ABOUT
Mastering communication between synthetic or hybrid materials with tissues is one of the grand challenges of contemporary biomedical systems that our society demands.
We want to uncover design rules to develop materials capable of communicating with living matter —pathogens, cells, tissues— and directing its behavior in a self-regulated manner to enable new biomaterials, therapeutics, and medical devices.
Biosensors fail to accurate diagnose diseases. Orthopedic implants cause inflammation and often rejection. Bacteria colonize wound dressings, catheters, and implants with devastating outcomes. Bacteria have grown resistant to many therapies. The surface of oxygenator membranes activate coagulation posing serious risks of thrombosis and stroke. These and many more examples have in common that the interface of the synthetic material is not sufficiently compatible resulting in deleterious signals to the living counterpart.
Nature blueprints are our inspiration
Like a master engineer, nature assembles its building blocks, molecules, in several levels of hierarchy from where function emerge. All the information necessary for this assembly is encoded in the molecular structure. We are inspired by natures’ and use its design principles to create materials that seamlessly interface with living matter to develop new paradigms for biomedicine.
RESEARCH LINES
MOLECULAR ENGINEERING
We are excited about using nature’s blueprints to make new molecules like “Lego” pieces that assemble into structures with totally new functions. Our synthetic palette is built from various types of advanced controlled radical polymerizations, click ligations, and supramolecular chemistry. We like to focus on water-soluble and amphiphilic molecules because of the unique aspects of the assembly in aqueous environments. Our box of toys includes multiblock copolymers, combs, stars, brushes, protein-polymer hybrids, dendrimers, and arborescent polymers.
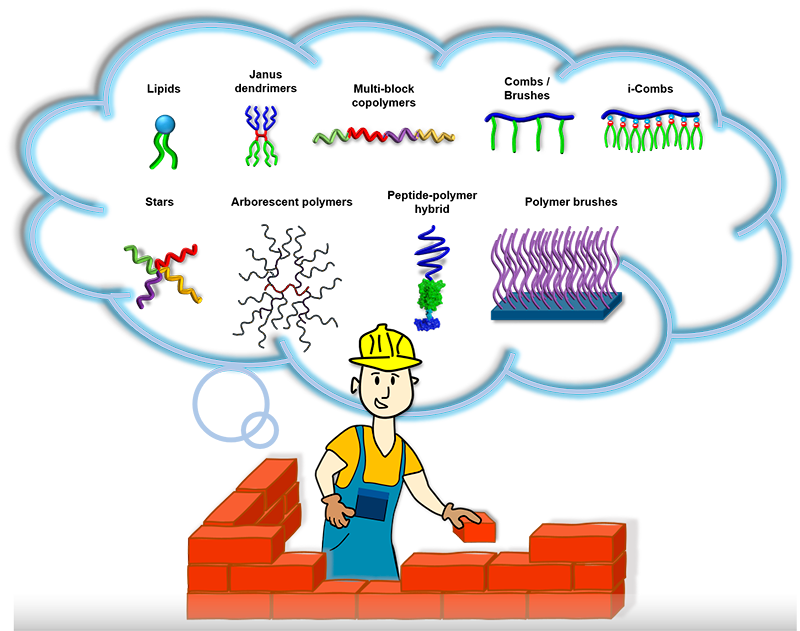
Macromolecular engineering: We synthesize macromolecular building blocks and program in their molecular structure their assembly and function
STEALTH AND ADAPTIVE BIOINTERFACES
Our goal is to develop adaptive coatings that interact and direct the biological surrounding in a self-regulated manner and their translation to medical and diagnostic applications. A cornerstone for this goal is to have selectivity, which means to be invisible to unwanted interactions while enhancing the specific ones. We are specialists in building “cloaks of invisibility”. These are nanoscale coatings that protect the surface against molecular fouling as well as bacterial colonization. They are also very important because they conceal the presence of synthetic materials.
But invisibility is not enough, so we decorate the surfaces with biomolecules and create feedback loops with the surrounding.
Examples:
- Optical affinity biosensors for the early diagnosis of (rare) diseases.
- Coatings that can sense the state of blood and selectively interfere with coagulation but just locally without affecting global hemostasis.
- Kill&Repel coatings that simultaneously prevent cellular adhesion and kill bacteria using mechanisms are selective and do not generate the emergence of resistance.
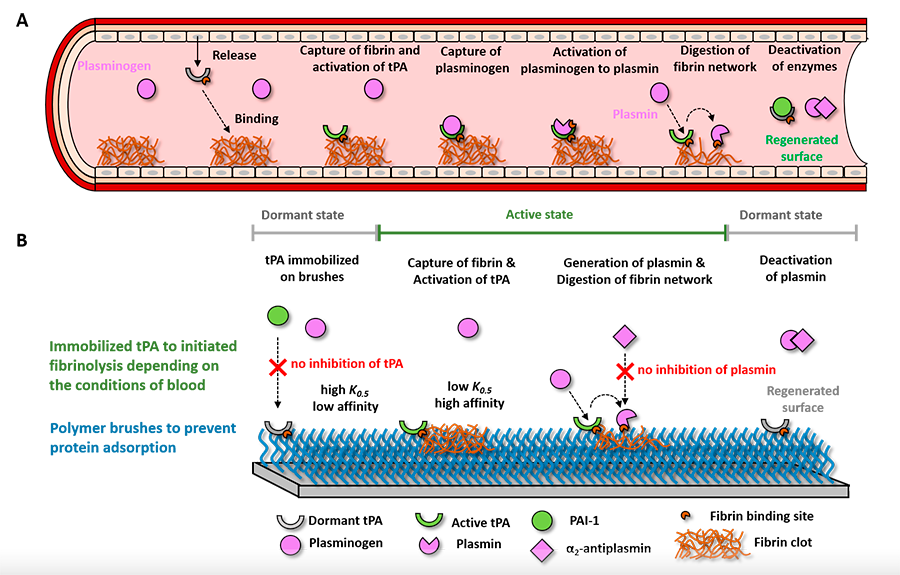
(A) Nature’s way to control the presence of clots inspires us to develop (B) adaptive hemocompatible coatings that in normal conditions are dormant and invisible to blood but that the presence of a dangerous clot causes its activation. In an active state, the coating directs blood to digest the clot thereby protecting the surface of the material.
Video 1. A macroscopic clot being digested by a fibrinolytic coating. DOI: 10.1021/acsami.1c01079

Kill&Repel coatings. The nanoscale coating selectively kills bacteria using the same weapons as bacteriophages and prohibits the adsorption of the debris of the dead bacteria. (DOI: 10.1002/adfm.202106656)
CELL MEMBRANE MIMICS AND PROTOCELLULAR SYSTEMS
We aim at building “quasi-living” synthetic cells (SynCells) by assembling non-living modules into micro-compartments displaying similar functionality and adaptivity as found in natural cells. This research holds promise to unveil some of the most daunting questions related to the origin of life, the transition from inanimate to living, and the emergence of diseases. We are not constrained to simply copy the current life forms which enable us to engineer synthetic cells with biologically inspired but augmented or even completely new functions to open new horizons for biomedicine, sensing, and therapeutics.
We are developing new concepts for cell membrane from non-natural amphiphiles with unprecedented mimicry to natural membranes but enhanced stability and which can be programmed to incorporate natural components of the cell membrane such as lipids, glycans, transmembrane proteins, etc. We enhance their livingness by hijacking functions of natural cells. For example by fusing the SynCells with bacteria or eukaryotic cells we integrate part of the complex molecular makeup of their membrane or by integrating active cell machinery, such as the divisome. We also investigate the introduction of superselectivity at the cell membrane to enable SynCells to recognize specific cells and actuate them. In particular, we are developing Synthetic Phagocytic Cells capable of engulfing and destroying pathogenic viruses and bacteria.
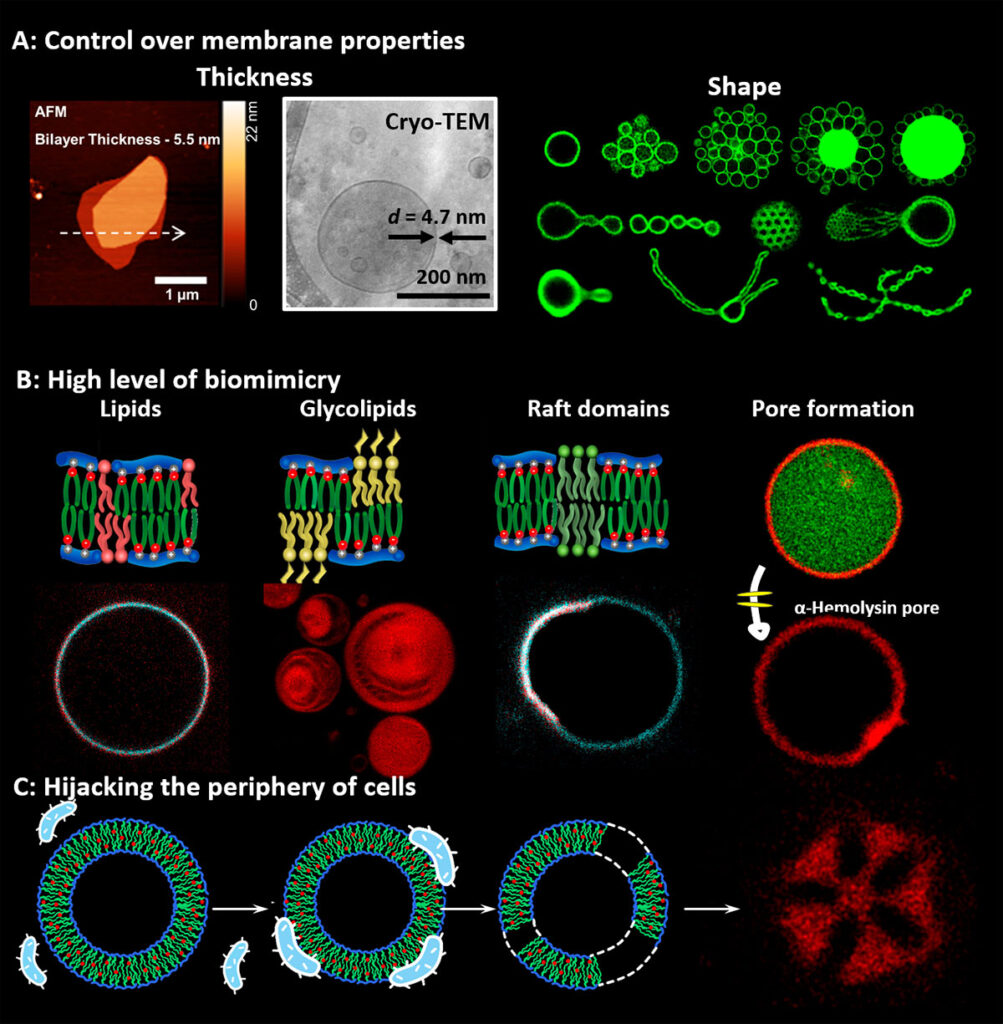
Examples of synthetic biomembranes. (A) We build vesicles from Janus dendrimers and amphiphilic comb polymers that accurately mimic the thickness, flexibility, and lateral mobility of natural membranes and to which we can program their shape. (B) The high biomimicry enables the incorporate functional lipids, glycolipids, and transmembrane proteins and to (C) form fuse with living cells to form hybrids of bacteria and synthetic cells.
TRANSLATION
We collaborate with hospitals and biomedical companies to translate our technologies to biomedical applications. This includes optical affinity biosensors for the early diagnosis of diseases, active non-thrombogenic coatings for extracorporeal membrane oxygenators, coatings for orthopedic implants, and new concepts for wound dressings that simultaneously protect the wound from bacteria and stimulate its healing.
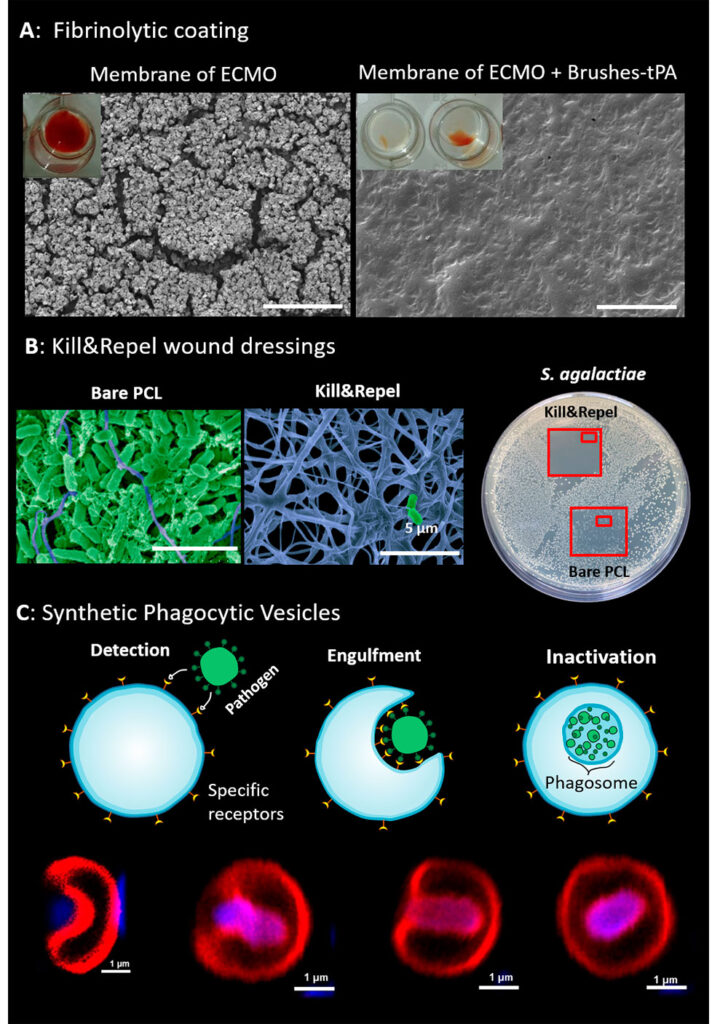
(A) Fibrinolytic adaptive coatings protect the membrane of oxygenators by digesting clots. (B) Kill&Repel coatings for wound dressing prevent the colonization of pathogens and disinfect the wound using powerful endolysins inspired by the antimicrobial activity of bacteriophages. (C) Synthetic phagocytic vesicles engulf and destroy bacteria and viruses.
STAFF
César Rodriguez-Emmenegger
PUBLICATIONS
See full publication list in Google Scholar
Check for more detailed information on the outputs of the Group at IBEC CRIS portal.
Publications list:
COLLABORATIONS
Prof. Virgil Percec (UPENN)
Prof. Andreas Herrmann (DWI)
Prof. Ulrich Schwaneberg (DWI)
Prof. Igor Potemkin (MSU)
PD. Dr. Tamás Haraszti (DWI)
Prof. Petra Schwille (MPI)
PD. Dr Rumiana Dimova (MPI)
Prof. Mike Klein (Temple U.)
Prof. Christopher Barner-Kowollik (QUT)
Prof. Gerhard Gompper (Jülich)
Prof. Gerard Lligadas (URV)
PD. Dr. Dr. Michael Hirtz (KIT)
Prof. Keith E. Cook (CMU)
Prof. Dr. Jakub Dostalek
Dr. Tomas Riedel (IMC)
PD. Dr. Oliver Grottke (UKA)
Prof. Filipe Mergulhão (LEBAPE)
NEWS

El IBEC y el Hospital West China celebran en Barcelona su tercera Conferencia conjunta de Medicina de Precisión
Esta semana ha tenido lugar en Barcelona la tercera Conferencia de Medicina de Precisión IBEC-WCH, un encuentro que consolidó la alianza estratégica entre el Instituto de Bioingeniería de Cataluña (IBEC) y el Hospital West China de la Universidad de Sichuan (WCHSU). La delegación china visitó España del 26 al 29 de noviembre, participando en un programa intensivo de actividades científicas, institucionales y de intercambio entre ambos centros.

Bioingeniería para la medicina de precisión en el 18º Simposio del IBEC
El 18º Simposio anual del IBEC se centró en ‘Bioingeniería para la Medicina de Precisión’, una de las áreas clave de aplicación del IBEC. Fueron cerca de 300 las personas asistentes al evento, entre las que se encontraba personal investigador local e internacional. Un ambiente multidisciplinar en el que expertos y expertas de otros centros y la propia comunidad del IBEC tuvieron la oportunidad de presentar sus proyectos e intercambiar conocimiento.

El IBEC y el UNIST organizan un foro sobre bioingeniería de última generación para reforzar la colaboración internacional
Hoy se ha celebrado el Foro sobre Bioingeniería de Última Generación, organizado por el Instituto de Bioingeniería de Cataluña (IBEC) y el Instituto Nacional de Ciencia y Tecnología de Ulsan (UNIST) de Corea del Sur. El evento ha puesto de relieve la investigación de vanguardia en bioingeniería y ha reforzado los lazos de colaboración entre ambas instituciones.

El IBEC y el ICMS se reencuentran en Eindhoven para celebrar su Simposio Anual en colaboración
Hoy, 24 de marzo, se ha celebrado el simposio conjunto ICMS-IBEC-MPIP. Un evento coorganizado por el Instituto de Bioingeniería de Cataluña (IBEC), el Instituto de Sistemas Moleculares Complejos (ICMS) y el Instituto Max Planck de Investigación en Polímeros (MPIP). Durante la jornada, investigadores de los tres centros han compartido sus áreas de investigación, buscando fortalecer los lazos científicos entre las instituciones.

El IBEC destaca en el BIST Forum con 4 proyectos BIST Ignite y un BIST Ignite Award
Hoy se ha celebrado el BIST Forum, un evento que reúne a la comunidad científica del BIST y que este año se ha centrado en la iniciativa conjunta de los siete centros CERCA para impulsar la medicina de precisión en el envejecimiento saludable. Durante el acto, se han anunciado los nuevos proyectos BIST Ignite para fomentar la investigación multidisciplinar, con la participación del IBEC en cuatro de los cinco seleccionados. Además, uno de los proyectos con participación del IBEC ha sido galardonado con un BIST Ignite Award.

El IBEC y el Hospital Sant Joan de Déu refuerzan su colaboración con una jornada de innovación traslacional
El Instituto de Bioingeniería de Cataluña y el Hospital Sant Joan de Déu han celebrado una jornada conjunta para impulsar colaboraciones en bioingeniería y medicina traslacional. El evento, realizado esta mañana en el IBEC, puso de relieve proyectos innovadores, presentó un programa de doctorado conjunto y fomentó el intercambio de ideas entre investigadores de ambas instituciones.

El científico del IBEC César Rodríguez-Emmenegger, galardonado con una prestigiosa subvención ERC Consolidator Grant
El investigador principal del IBEC César Rodríguez-Emmenegger ha sido galardonado con una subvención “ERC Consolidator Grant”. Esta prestigiosa financiación europea apoya a los científicos en la fase de consolidación de sus equipos de investigación, permitiéndoles perseguir ideas científicas innovadoras. 2 millones de euros a lo largo de cinco años permitirán a Rodríguez-Emmenegger y su equipo desarrollar células sintéticas fagocíticas (PSC) para combatir patógenos resistentes a los antibióticos.

El IBEC y el VHIR celebran una jornada de colaboración para fomentar las sinergias
La 1ª Jornada Colaborativa Traslacional entre el Vall d’Hebron Instituto de Investigación (VHIR) y el Instituto de Bioingeniería de Cataluña (IBEC), celebrada el 21 de noviembre, ha sido una oportunidad para conocer proyectos y líneas de investigación de ambas instituciones y promover la interacción entre los profesionales.

El IBEC y el Banco de Sangre y Tejidos fortalecen lazos con una jornada de colaboración traslacional
El IBEC y el Banc de Sang i Teixits han celebrado una jornada para explorar nuevas colaboraciones en bioingeniería y medicina traslacional. El encuentro, celebrado ayer en el IBEC, destacó proyectos innovadores, presentó un programa de doctorado conjunto y reforzó la conexión entre investigación biomédica y aplicaciones clínicas.
JOBS

Senior Researcher at the Bioinspired Interactive Materials and Protocellular Systems Research Group
Ref: SR-CR-E //Deadline: 16/11/2025

Postdoctoral researcher at the Bioinspired Interactive Materials and Protocellular Systems Research Group
Ref: PD- CR // Deadline: 25/07/2025

Postdoctoral researcher at the Bioinspired Interactive Materials and Protocellular Systems Research Group
Ref: PD-CR // Deadline: 10/03/2025

Postdoctoral researcher at the Bioinspired Interactive Materials and Protocellular Systems Research Group
Ref: PD_CR // Deadline: 10/10/2023

Predoctoral researcher at the Bioinspired Interactive Materials and Protocellular Systems Research Group (PhD-CR)
Reference: PhD-CR // Deadline: 07/07/2023

Predoctoral researcher at the Bioinspired Interactive Materials and Protocellular Systems Research Group
Ref: PhD-CR // Deadline: 19/05/2023


 ibecbarcelona.eu
ibecbarcelona.eu