The Nanomalaria group is a joint unit affiliated with IBEC and the Barcelona Institute for Global Health (ISGlobal), located in the Esther Koplowitz Centre near Hospital Clínic (Barcelona).
About
The current activity of the Nanomalaria group is focused on the development of nanomedicine-based systems to be applied to malaria prophylaxis, diagnosis and therapy.
Methods for the diagnosis of malaria can benefit from nanotools applied to the design of microfluidic-based devices for the accurate identification of the parasite’s strain, its precise infective load, and the relative content of the different stages of its life cycle, whose knowledge is essential for the administration of adequate therapies.
Malaria is arguably one of the main medical concerns worldwide because of the numbers of people affected, the severity of the disease and the complexity of the life cycle of its causative agent, the protist Plasmodium spp. The clinical, social and economic burden of malaria has led for the last 100 years to several waves of serious efforts to reach its control and eventual eradication, without success to this day.
With the advent of nanoscience, renewed hopes have appeared of finally obtaining the long sought-after magic bullet against malaria in the form of a nanovector for the targeted delivery of antimalarial drugs exclusively to Plasmodium-infected cells. Nanotechnology can also be applied to the discovery of new antimalarials through single-molecule manipulation approaches for the identification of novel drugs targeting essential molecular components of the parasite.
The benefits and drawbacks of these nanosystems have to be considered in different possible scenarios, including economy-related issues that are hampering the progress of nanotechnology-based medicines against malaria with the dubious argument that they are too expensive to be used in developing areas. Unfortunately, it is true that the application of nanoscience to infectious disease has been traditionally neglected, with most research resources overwhelmingly biased towards other pathologies more prominent in the developed world. Thus, extra ingenuity is demanded from us: malaria-oriented nanomedicines not only need to work spotless; they have to do so in a cost-efficient way because they will be deployed in low-income regions.
The driving force of the Nanomalaria group is our personal commitment to applying nanomedicine to infectious diseases of poverty through several research lines:
- Exploration of different types of encapsulating structure (liposomes, synthetic and natural polymers), targeting molecule (protein, polysaccharide, nucleic acid aptamers), and antimalarial compound (e.g. new structures derived from marine organisms and antimicrobial peptides) for the assembly of nanovectors capable of delivering their drug cargo with complete specificity to diseased cells.
- Study of metabolic pathways present in Plasmodium but absent in humans, with the aim of identifying specific enzymes as therapeutic targets.
- Use of glycosaminoglycans for innovative antimalarial strategies.
- Design of new methods for the targeted drug delivery to Plasmodium stages in the mosquito vector.
- Investigation of novel drugs against insect-borne diseases working through radically new mechanisms.
- Extension of our activities to new pathologies (leishmaniasis).
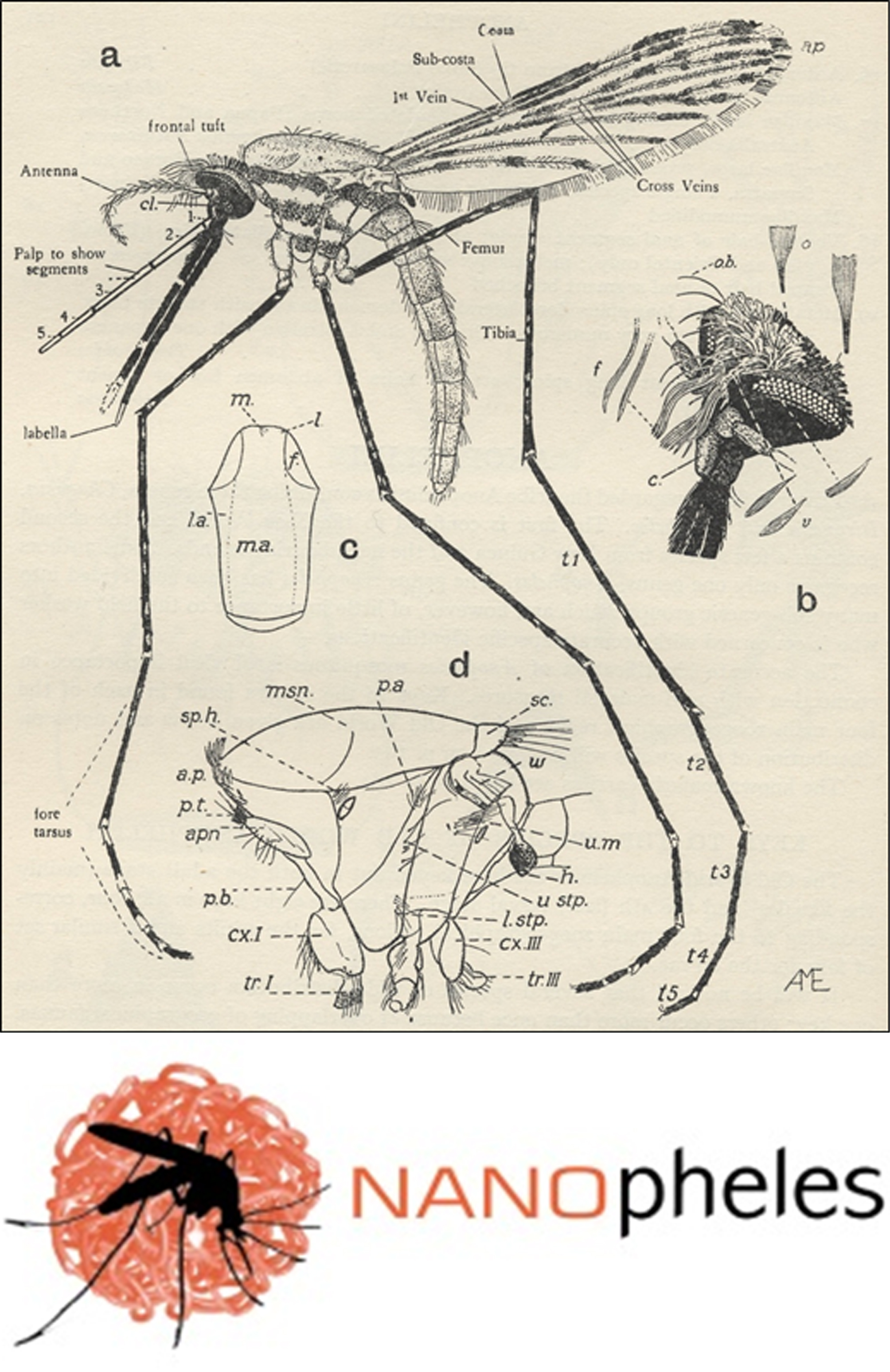
FIGURE 1. Top: female Anopheles gambiae mosquito. From: John Smart, A Handbook for the Identification of Insects of Medical Importance, British Museum, London, 1948. Bottom: Logo of the NANOpheles project (EURONANOMED III call) coordinated by the Nanomalaria Group.
FIGURE 2. Cover image of the PhD Thesis of Dr. Elisabet Martí Coma-Cros, Investigation of branched and linear polymers as oral delivery systems of antimalarial drugs. 2019. Universitat de Barcelona. Cover design by Mar Martí Coma-Cros.
Staff
Xavier Fernàndez-Busquets
Projects
| NATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| PROTAGINforMAL Characterization of the activity of the protein aggregation inhibitor YAT2150 as new antimalarial drug mechanism (2022-2025) | MICIU, Generación Conocimiento: Proyectos I+D | Xavier Fernández- Busquets |
| DAMPA Development of a new generation of antimalarial drugs for the post-artemisinin era (2022-2024) | MICIU, Proyectos Pruebas de Concepto | Xavier Fernández- Busquets |
| PRIVATELY-FUNDED PROJECTS | FINANCER | PI |
|---|---|---|
| CATMAL Compact Analytical Tool for robust MALaria decentralized diagnosis and community surveillance (2023-26) | Obra Social La Caixa, CaixaResearch Health | Xavier Fernández- Busquets |
| FINISHED PROJECTS | FINANCER | PI |
|---|---|---|
| Coated liposome nanocomplexes as drug delivery systems for treatment of leishmaniasis (2019-2023) | Fundació La Marató de TV3 | Xavier Fernández- Busquets |
| Identificació de fraccions d’heparina com a noves teràpies antimalàriques (2016-2019) | Bioiberica, S.A. | Xavier Fernández- Busquets |
| IPANAT Investigación de la agregación proteica como nueva diana antimalárica (2019-2021) | MICIU, Retos investigación: Proyectos I+D | Xavier Fernández-Busquets |
| NANOpheles Development of nanovectors for the targeted delivery in Anopheles mosquitoes of agents blocking transmission of Plasmodium parasites (2017-2021) | EURONANOMED III: European innovative research & technological development projects in nanomedicine | Xavier Fernández- Busquets |
| PaMapDX · Pan-Malaria Aptamer-based Rapid Diagnostic Test (2020-2021) | Obra Social La Caixa, Caixaimpulse | Elena Lantero |
| NANOMISSION Ingeniería de nanovectores para la liberación de fármacos antimaláricos a fases de transmisión de Plasmodium | MINECO, Retos investigación: Proyectos I+D | Xavier Fernández- Busquets |
| NANOMALNET Exploración de nuevas moléculas direccionadoras eficientes para la liberación de antimaláricos | Biotechnology Programme, MINECO, Spain (BIO2011-25039) | Xavier Fernández- Busquets |
| Amphoteric polyamidoamines as innovative tools to selectively direct antimalarial drugs towards Plasmodium-infected red blood cells | Fundación CARIPLO | Xavier Fernández- Busquets |
Publications
Check for more detailed information on the outputs of the Group at IBEC CRIS portal.
Publications list:
Equipment
- Zeiss Primostar microscope
- Shake ‘N’ Stack (Thermo Hybaid) hybridization oven
- Rotatory evaporator RS 3000-V (Selecta)
- Plasmodium falciparum cell cultures
Collaborations
- Prof. Dario Anselmetti
Universität Bielefeld, Germany. Single molecule force spectroscopy - Prof. Maria Antònia Busquets
University of Barcelona, Spain - Prof. Elisabetta Ranucci
Università degli Studi di Milano, Italy - Prof. José Manuel Bautista
Universidad Complutense de Madrid, Spain - Dr. Matthias Rottmann
Swiss Tropical and Public Health Institute, Basel, Switzerland - Prof. Robert Sinden
Imperial College London, UK - Dr. Israel Molina
Hospital Universitari Vall d’Hebron, Barcelona - Prof. José Luis Serrano
Instituto de Nanociencia de Aragón, Zaragoza - Prof. Johan Engbersen
University of Twente, The Netherlands - Dr. Santiago Imperial
University of Barcelona, Spain - Dr. Eduardo Prata Vilanova
Universidade Federal do Rio de Janeiro, Brazil. Exploration of sulfated polysaccharides of marine origin as antimalarials - Dr. Maria Manconi
Università de Cagliari, Sardinia, Italy. Liposome technology - Dr. Krijn Paaijmans
CRESIB, Barcelona, Spain - Dr. Ellen Faszewski
Wheelock College, Boston, USA. Marine sponge cell adhesion - Prof. Bernard Degnan
University of Brisbane, Australia - Dr. Francisco J. Muñoz
Parc de Recerca Biomèdica de Barcelona, Spain. Amyloid diseases - Dr. Inga Siden-Kiamos
FORTH Institute of Molecular Biology & Biotechnology, Greece. Development of the malaria parasite within the mosquito - Prof. Salvador Ventura
Universitat Autònoma de Barcelona, Bellaterra, Spain. Aggregative proteins - Dr. Juan José Valle-Delgado
Aalto University, Helsinki, Finland. Atomic force microscopy - Prof. Mats Wahlgren
Karolinska Institutet, Stockholm, Sweden - Dr. Fatima Nogueira
Instituto de Higiene e Medicina Tropical, Lisboa, Portugal. Antimalarial drug assays in Plasmodium-infected mosquitoes and mice. - Dr. Christian Grandfils
University of Liège, Belgium. Biomaterials research. - Salvador Borros
Institut Químic de Sarrià, Barcelona. Materials Chemistry - Paula Gomes
Universidade do Porto, Portugal. Development of new antimalarial drugs - José Antonio García Salcedo
Instituto de Parasitología y Biomedicina “López-Neyra”, Consejo Superior de Investigaciones Científicas (CSIC), Granada, Spain. Synthesis of chitosan nanoparticles - Eva Baldrich
Hospital Universitari Vall d’Hebron, Barcelona. Malaria diagnosis - Kim Williamson
Uniformed Services University of the Health Sciences, Bethesda, USA. Basic biology of bacterial, viral, and parasite diseases - Teresa Sierra
Instituto de Nanociencia de Aragón, Zaragoza, Spain. Dendrimer technology - Jos Paulusse
University of Twente, The Netherlands. Encapsulation of peptides in tailor-made multifunctionalized nanocarriers and polyamidoamine-derived nanogels
News
Alterar el control de proteïnes del paràsit pot obrir noves vies terapèutiques contra la malària
Investigadors de l’IBEC i ISGlobal han dirigit un estudi que apunta a l’agregació de proteïnes com a possible diana per a trobar noves maneres de reduir la viabilitat del Plasmodium falciparum, el principal agent causant de la malària. En induir l’agregació proteica, van observar trastorns considerables en l’homeòstasi de les proteïnes i una reducció significativa del creixement del paràsit. Els resultats situen el control de l’agregació proteica com una diana prometedora per a les teràpies antimalàriques.

Tres investigadors de l’IBEC seleccionats en la convocatòria Caixa Research 2024 per liderar projectes de recerca en salut
Els investigadors de l’IBEC Benedetta Bolognesi, Xavier Fernàndez Busquets i Xavier Trepat han estat seleccionats en la convocatòria CaixaResearch 2024 per liderar sengles projectes de recerca. Les ajudes rebudes suposen un suport econòmic perquè els científics duguin a terme la seva recerca en àrees clau com el càncer, la malària i l’esclerosi lateral amiotròfica.

Científics de l’IBEC i ISGlobal desenvolupen un compost innovador eficaç contra malària i la leishmaniosi
Un estudi liderat per l’IBEC i ISGlobal ha demostrat el potencial antileishmanial d’un compost antimalàric. Dissenyat inicialment per a la malària, aquest fàrmac mostra una elevada eficàcia contra la leishmaniosi, marcant un avenç únic i prometedor per al tractament d’ambdues infeccions.

Predoctoral researcher at the Nanomalaria research group
Introduction to the vacant position: The Nanomalaria Group is looking for an Early Stage Researcher (PhD student) to develop his/her PhD thesis project on the development of new antimalarial drugs. … Read more

Últims avenços de la nanomedicina europea contra la malària explicats en un nou vídeo
La malària mata més de 500.000 persones cada any. En els darrers tres anys, els investigadors del Projecte NANOpheles van treballar en el desenvolupament de nanovectors dirigits als paràsits Plasmodium dins del mosquit vector. Xavier Fernàndez-Busquets, coordinador del projecte finançat per la UE, explica els avenços en un nou vídeo.
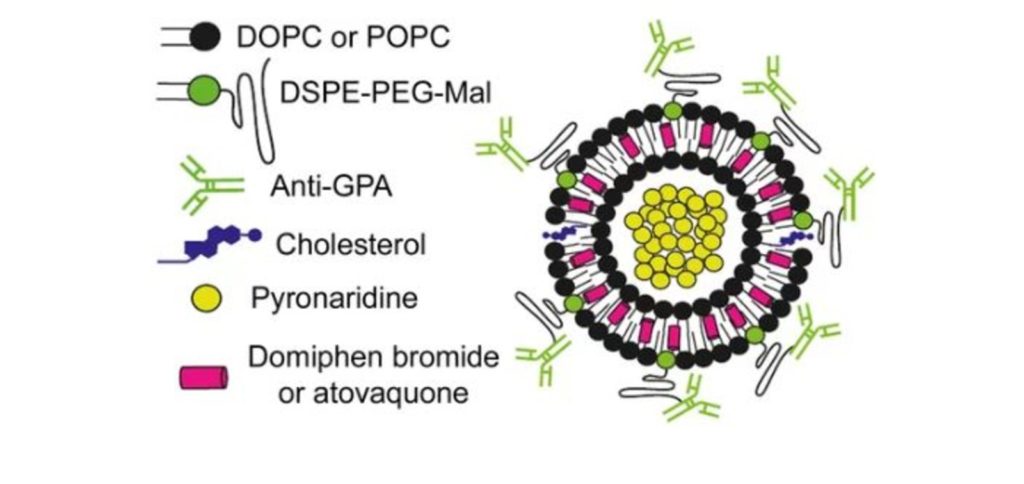
Els nanovectors podrien millorar l’administració combinada de fàrmacs contra la malària
Segons indica l’estudi, l’estratègia té, a més, l’avantatge de reconèixer al gametocist, la fase transmissible del paràsit. Encapsular dos fàrmacs amb propietats diferents en nanovesícules envoltades per anticossos millora notablement la seva especificitat i eficàcia, segons un estudi liderat per Xavier Fernández-Busquets, director de la unitat mixta de Nanomalària de l’Institut de Bioenginyeria de Catalunya (IBEC) i l’Institut de Salut Global de Barcelona (ISGlobal), centre impulsat per ”la Caixa”. La combinació de dos fàrmacs que difereixen en el seu mecanisme d’acció és la base de les teràpies més reeixides avui dia per tractar la malària. Tot i això, la diferència en propietats fisicoquímiques dels fàrmacs (solubilitat, vida mitjana, etc.) afecta moltes vegades a l’eficàcia del tractament.
Jobs
Predoctoral Researcher at the Nanomalaria Group
Ref: PHD_XF // Deadline: 05/10/2025
Research Assistant at the Nanomalaria Research Group
Ref: RA-XF// Deadline: 15/06/2023
Postdoctoral researcher at the Nanomalaria Research Group (Ref: PD-XF).
Ref: PD_XF // Deadline: 03/03/2023
Postdoctoral researcher at the Nanomalaria Research Group (Ref: PD-XF).
Ref: PD-XF / Deadline: 13/03/2023
Postdoctoral Researcher at the Nanomalaria Research Group
The Nanomalaria Group is looking for a Postdoctoral Researcher to work on the development of the new antimalarial drug YAT2150. The contract will be within the framework of one of … Read more
Predoctoral researcher at the Nanomalaria research group
Introduction to the vacant position: The Nanomalaria Group is looking for an Early Stage Researcher (PhD student) to develop his/her PhD thesis project on the development of new antimalarial drugs. … Read more
Postdoctoral researcher at Nanomalaria Research Group
Application Deadline: 15/07/2021Ref: PD-XF The Nanomalaria group at the Institute for Bioengineering of Catalonia (IBEC) offers a Postdoctoral position.


 ibecbarcelona.eu
ibecbarcelona.eu