ABOUT
The inability of the central nervous system (CNS) to regenerate has been attributed to several factors: First, the natural formation of cellular “bridges” essential for axonal regeneration does not occur at the site of injury. Second, the injured CNS fails to produce growth factors necessary to stimulate regeneration. Third, neurons in the injured CNS do not adequately initiate a “growth program” necessary for new regeneration. Finally, the environment of the injured adult spinal cord presents obstacles to regeneration, specifically due to inhibitory extracellular matrix molecules that accumulate around the injury site and the presence of inhibitory proteins in adult myelin that hinder regeneration.
We are committed to elucidating the molecular processes underlying neuronal regeneration and using these insights to develop practical strategies for repairing damaged CNS circuits.
Our research covers a broad spectrum from the identification of ECM molecules that impede axon regeneration to the development and application of advanced biomimetic materials in spinal cord injury (SCI) models.
Our group is interested in elucidating the molecular mechanisms governing regenerative failure after central nervous system injury and leveraging this knowledge to develop rationally tuned biomaterial strategies to reverse paralysis. Our goal is to translate these findings to humans.
RESEARCH FOCUS
Understanding the Barriers to Regeneration:
The primary focus of this research is to understand the role of the extracellular matrix (ECM) in spinal cord injury and regeneration.
Using quantitative mass spectrometry proteomics, our team is characterizing the ECM and ECM-associated proteome in spinal cords across developmental stages and injury conditions. This approach allows the identification of specific ECM components that change after injury, providing insight into potential therapeutic targets.
The comprehensive proteomic profile is further enhanced by transcriptomic profiling to identify soluble components, cellular contributors, and spatial information. This in-depth analysis of the ECM in both healthy and injured states aims to unravel the complex interactions and changes that occur and to guide the development of strategies to modulate the ECM to promote regeneration
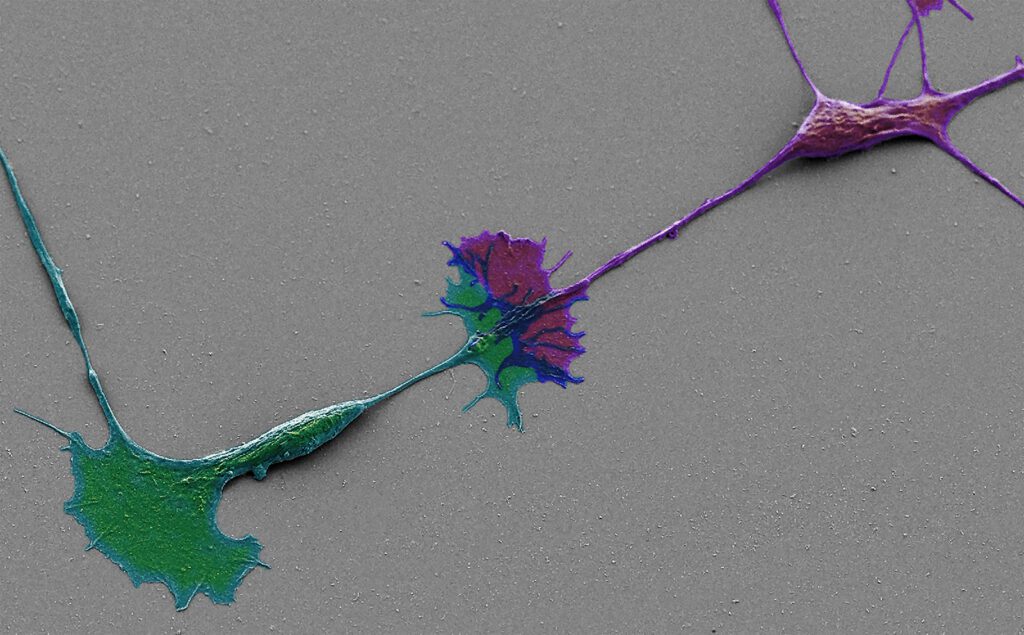
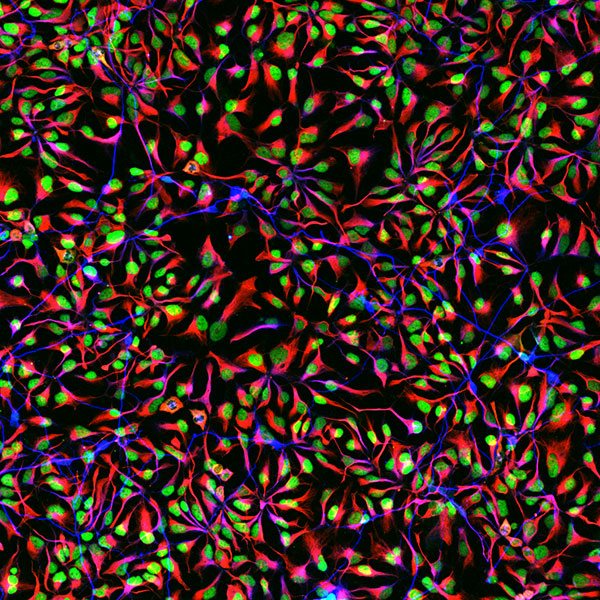
Human spinal motor neurons grown on artificial matrix. SEM image of neurons interacting through their growth cones. Cells are falsely colored in green and purple.
New tools to study injury and regeneration in vitro:
This research area uses cutting-edge bioengineering methods and three-dimensional systems to mimic spinal cord structures and analyze their responses to injury in the laboratory. It uses two primary techniques:
1. Human Spinal Cord Organoids (hSCOs): hSCOs are generated using 3D printed organoid-on-a-chip technology, which creates a tubular shape that mimics the geometry of the spinal cord. This structure allows us to study how the shape of the tissue affects cellular organization within the organoids. In addition, by incorporating ECM signals at specific times after spinal cord injury, the study aims to explore the evolving nature of spinal cord injury and evaluate treatments based on ECM components identified through this research.
2. 3D printed human spinal cord constructs: This method combines two sophisticated printing technologies – cell bioprinting and volumetric printing – to produce a detailed, four-dimensional model of the human spinal cord with precise accuracy. The focus is on faithfully reproducing the white and gray matter of the spinal cord. It uses an exoskeletal framework to strategically place each bioprinted cell, closely mimicking the architecture of the spinal cord. This approach is designed to provide insight into the dynamic functional behavior of the spinal cord in vitro.
Translational Strategies for the Treatment of Spinal Cord Injury:
The third area of our research focuses on the development of innovative and effective biomaterial-based therapies for spinal cord injury (SCI) using in vivo models. Our team is implementing two different approaches depending on the stage of injury:
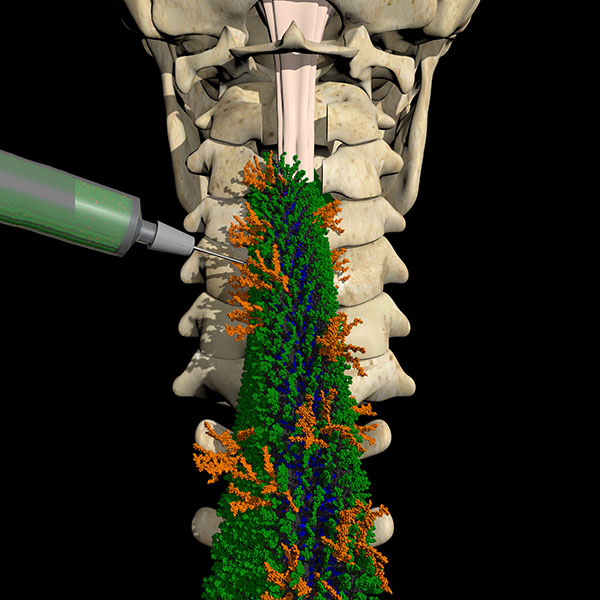
1. Injectable functionalized synthetic hydrogels for acute injury: We are creating chemically defined hydrogels that mimic various ECM signals, specifically tailored to treat the early stages of spinal cord injury. The goal is to study their impact on factors such as inflammation, cell survival, blood vessel formation, glial scar formation, and nerve fiber regeneration
2. 3D printed spinal cord constructs for chronic injury: For more advanced chronic spinal cord injuries, we are developing artificial 3D constructs embedded with ECM signals and living cells. These constructs are designed to provide nutritional support, promote neural growth, and support the functional development of native cells in nearby tissues. The primary goal is to enhance the integration, maturation, and connectivity of transplanted cells, which is critical for restoring motor function in models of chronic spinal cord injury.

STAFF
Staff members
PROJECTS
Mobile Scaffolds as a Therapeutic Target to Repair Spinal Cord Injury.
Project Reference: L31CVNORTHU
Principal Investigator: Zaida Alvarez
Dates: 11/2022-11/2025
Financial Entity: Northwestern University
Defining the Matrisome of Spinal Cord Injury to Design New Experimental Treatments.
Project Reference: CNS2022-135407
Principal Investigator: Zaida Alvarez
Dates: 09/2022-09/2025
Financial Entity: Spanish Ministry of Science
Mobile Hydrogels for Enhancing the Bioactivity of Human Neural Progenitor Cells for Spinal Cord Injury Repair (MobiHydroSpine).
Project Reference: PID2021-124839OA-I00
Principal Investigator: Zaida Alvarez
Dates: 09/2022-09/2025
Financial Entity: MICIU. Generación Conocimiento: Proyectos I+D Spanish Ministry of Science
FINALIZED:
3D printed ECM constructs as therapies to repair the injured spinal cord.
Principal Investigator: Zaida Alvarez
Dates: 2022
Financial Entity: Mike Lane-Castellers de la Vila de Gràcia.
PUBLICATIONS
EQUIPMENT
- IH-0415 Infinite Horizon IMPACTOR Mouse/Rat
- DigiGait Imaging System – Mouse Only with Incline
- WPI Stereotaxic for mice, Digi and Portable, SGL M
- Electrode Manipulator without 1770 holder
- UltraMicro Pump and SmartTouch Controller
- Solo Controller with 50mm Single Axis
- Manipulator Adapter for Stoelting stereotaxic
- Rod Holder For Motorized Manipulator
- Hamilton Syringes
COLLABORATIONS
- Samuel I. Stupp
Simpson Querrey Institute, Northwestern University - Aitziber López Cortajarena
CICbiomagune - Juan Alberto Ortega
Universitat de Barcelona - Evangelos Kiskinis
Northwestern University - Ivan R. Sasselli
CSIC - Maria Luisa Garcia Lopez
Universitat de Barcelona - Riccardo Levatto
Utrect University - Simone Di Giovani
Imperial College - Antonio Oliviero
Hospital Nacional de Parapléjicos de Toledo
NEWS
Una nanoteràpia recoberta de sucre protegeix les neurones en malalties neurodegeneratives
El nou tractament, basat en nanofibres i trehalosa, un sucre natural de les plantes, atrapa i neutralitza les proteïnes tòxiques per aturar la progressió de la malaltia. Un cop atrapades, les proteïnes tòxiques ja no poden penetrar en les neurones i es degraden sense causar danys. L’estudi, publicat a la revista Journal of the American Chemical Society ha estat desenvolupat per científics de l’Institut de Bioenginyeria de Catalunya (IBEC) i la Northwestern University, als Estats Units.

L’IBEC rep finançament dels Instituts Nacionals de Salut dels Estats Units per avançar en la regeneració neural
La investigadora principal de l’IBEC, Zaida Álvarez, ha rebut finançament els Instituts Nacionals de Salut (NIH) dels Estats Units per desenvolupar models de medul·la espinal basats en cèl·lules mare. El projecte, coordinat per la Northwestern University amb la participació de l’IBEC i l’IDIBELL, permetrà estudiar malalties neurodegeneratives i desenvolupar nous tractaments per a lesions medul·lars i altres patologies neurodegeneratives.

Organoides de medul·la espinal per estudiar tractaments per a la paraplegia
Es tracta d’un projecte centrat en la creació d’un dispositiu imprès en 3D on es cultivarà un organoide de medul·la espinal humana per estudiar el dany medul·lar i el posterior testatge de fàrmacs. La recerca, liderada per la investigadora principal de l’IBEC Zaida Álvarez, ha rebut finançament part de la Fundació Internacional per a la Recerca de la Paraplegia.

L’IBEC es reforça amb tres nous grups de recerca en teràpies avançades i emergents
L’IBEC estrena el 2024 amb la incorporació de tres nous grups de recerca que seran liderats per Manuel Salmerón Sánchez, Zaida Álvarez Pinto i Xavier Rovira Clavé. Amb aquestes incorporacions, l’IBEC enforteix el seu posicionament en el camp de les teràpies avançades i emergents.
Investigadores de l’IBEC posen de manifest el paper de la dona en ciència en diferents mitjans
Coincidint amb la celebració del dia 11 de febrer, Dia de la Nena i la Dona en Ciència, Núria Montserrat és una de les tres protagonistes de la campanya de … Read more
Aconsegueixen neurones madures en el laboratori a partir de cèl·lules mare per poder estudiar malalties neurodegeneratives
Zaida Álvarez, investigadora de l’IBEC, i Alberto Ortega, investigador de la UB, han aconseguit les primeres neurones altament madures cultivades al laboratori, a partir de cèl·lules mare pluripotents, fent servir … Read more
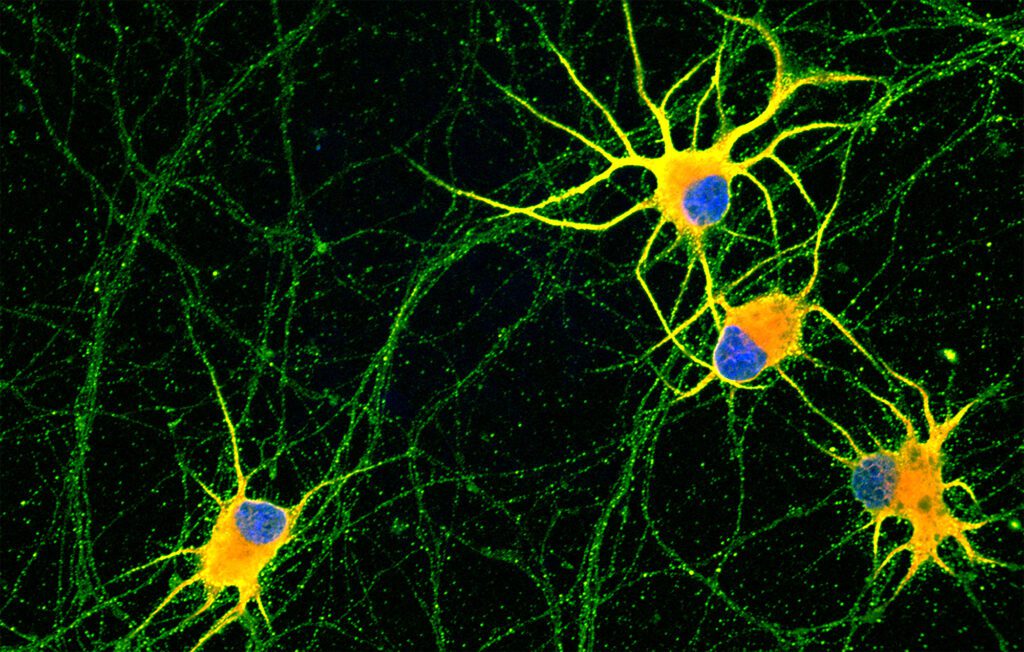
Investigadors aconsegueixen cultivar neurones madures al laboratori per estudiar malalties neurodegeneratives
Investigadors de l’Institut de Bioenginyeria de Catalunya (IBEC) i de la Universitat de Barcelona (UB) han aconseguit crear les primeres neurones altament madures a partir de cèl·lules mare pluripotents induïdes … Read more

Zaida Álvarez rep el premi Muy Nanotecnología i una menció especial del Premi Duran Farell de Recerca Tecnològica
El passat 29 de novembre, Zaida Álvarez, investigadora ‘Ramon y Cajal’ a l’Institut de Bioenginyeria de Catalunya (IBEC), va rebre el premi Muy Nanotecnología de mans de la ministra de … Read more
JOBS

Researcher in Training for Neural Regeneration Research Group
Ref: RT-ZA // Deadline: 12/03/2025

PhD at the Biomaterials for Neural Regeneration Research Group
Ref: PHD_ZA // Deadline: 10/03/2025

Research Assistant at the Biomaterials for Neural Regeneration Research Group
Ref: (RA_ZA)// Deadline: 30/10/2024

Laboratory technician at the Biomaterials for neural regeneration (LT_ZA)
Ref: LT_ZA // Deadline: 22/03/2023


 ibecbarcelona.eu
ibecbarcelona.eu
