Group: Cellular and molecular mechanobiology and Pluripotency for organ regeneration.
Group leader: Pere Roca-Cusachs (proca@ibecbarcelona.eu) and Núria Montserrat (nmontserrat@ibecbarcelona.eu)
Research project
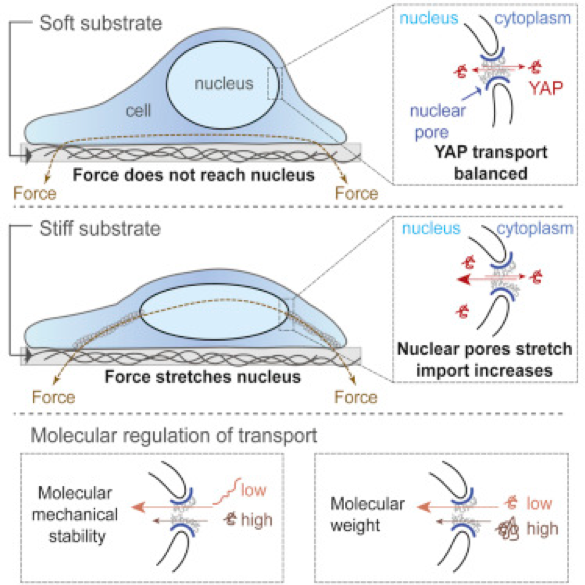
The Cellular and Molecular Mechanobiology lab at IBEC studies cell mechanical interactions with their environment. These physical interactions determine how cells proliferate, differentiate, and move, and regulate development, tumorigenesis, or wound healing. Just like biochemical stimuli initiate signaling cascades, mechanical forces affect the links and conformation of a network of molecules connecting cells to the extracellular matrix. Research in the group aims precisely at unraveling the mechanisms that these molecules use to detect and respond to mechanical stimuli like forces or tissue rigidity, triggering downstream cell responses. To this end, we combine biophysical techniques like magnetic tweezers, Atomic Force Microscopy, traction microscopy, and microfabricated force sensors with molecular biology, advanced optical microscopy, and theoretical modelling.
The Pluripotency for Organ Regeneration group is interested in providing fundamental knowledge on cell specification and differentiation. Through the use of pluripotent stem cells our aim is to dissect the biochemical and biophysical cues instructing cell fate decisions and to perturb those responses in order to mimic human disease and/or identify novel pathways during disease progression. Through the development of self-assembly organ-like cultures (so called organoids) we are able to interrogate for early cell fate decisions during the onset of renal, retinal and cardiac differentiation.
We take advantage of a transdisciplinary approach that includes: 1) the generation of human pluripotent stem cells (hPSCs) from patients (induced pluripotent stem cells); 2) the derivation of protocols for hPSCs differentiation through the emulation of biochemical and biophysical cues determinant for tissue development in vivo; 3) the massive application of genome editing, transcriptomics and tissue engineering approaches for understanding, perturbing and modulating all these processes.
Job position
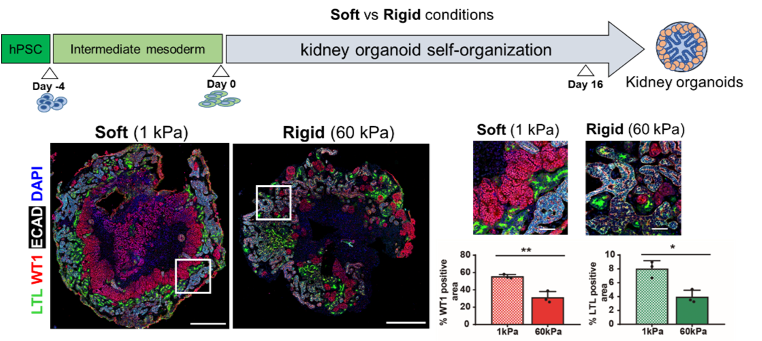
One of the main applications where mechanical factors are crucial is the generation of organ-like structures from human pluripotent stem cells (hPSCs), so called organoid cultures. Up to date the field is exploiting intrinsic characteristics of hPSCs (self-organization and symmetry breaking) using morphogens and cytokines that dictate hPSC differentiation. However, tissue development also responds to mechanical cues imposed by the tissue environment (i.e., folding, stretching, among many others). Our groups have shown that the generation of renal progenitor cells in a soft microenvironment results in the derivation of higher-grade kidney organoids (Nat. Mater. 2019, 18, 397–405). This finding, together with recent observations in the field prompt us to hypothesize that the use of mechanical stimuli alone or in combination with biochemical cues may lead to the generation of hPSC-organoids, exploiting their intrinsic capacity to self-organize and respond to different mechanical stimuli (i.e., stiffness of substrate, stretching, confinement, among others). Accordingly, we aim to interrogate how mechanic stimuli (substrate stiffness, stretching and compression) leads to changes at the transcriptomic (bulk RNA seq, scRNAseq) and epigenetic level (ChIP on ChIP for histone modifications genome wide and scATACseq). The identification of the mechanisms linking both cell mechanics and gene expression will be crucial to improve our understanding of tissue development and disease.
We are seeking for a curious PhD student with background in Biology, Biotechnology and/or Physics. We ensure that the Candidate will be exposed to cutting-edge techniques, including: human pluripotent stem cells-related in vitro techniques, organoid generation, genome editing, biomechanical techniques, computational modeling, and advanced microscopy. The PhD candidate will also have the opportunity to perform internships in top notch laboratories in the field of organoid engineering and cell mechanics.

