About
The “Biomimetic Systems for Cell Engineering” group is a multidisciplinary research group focussing its research activities on the development and application of new artificial systems that mimic tissue micro and nanofeatures for biomimetic in vitro assays.
The use of more biomimetic and complex 3D models in in vitro assays is now a hot and extremely relevant topic.
“Flat biology” results are questioned when being transferred to in vivo, while the pressure to decrease animal testing continues increasing. As a group of engineering providers working in the biotechnological field, we see these issues as both a challenge and a clear opportunity to impact the field with novel technological solutions. Our research ambition will be to develop better engineering tools to help in the development of organotypic cell culture models, easy to implement in daily cell culture routines, so they have a real and meaningful impact in the biotechnological arena and, therefore, will impact applications in basic cell research, disease modelling and regenerative medicine.
Along with this line, we are working towards the following objectives: (i) to engineer and validate a complex in vitro model of small intestinal epithelium, (ii) to validate a novel in vitro model of engineered cardiac tissue and (iii) to engineer a novel vessel-on-chip to reproduce the tumor metastatic environment. To carry out our research we employ cutting edge biofabrication technologies such as bioprinting and novel biological tools such as organoids.
Staff
Projects
| INTERNATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| GUT3D-PLATE · Ready-to-use cell culture plates for gut 3D models (2020-2022) | European Commission, ERC-PoC | Elena Martínez |
| COMIET · Engineering Complex Intestinal Epithelial Tissue Models (2015-2022) | ERC Consolidator Grant | Elena Martínez |
| BRIGHTER · BIOPRINTING BY LIGHT-SHEET LITHOGRAPHY (2019-2023) | European Commission FET Open | Elena Martínez |
| PRIVATELY FUNDED PROJECTS | FINANCER | PI |
|---|---|---|
| PROMISE · BioPrinted hydROgel MicrofluIdicS to mimic patient-specific tumor mEtastatic microenvironment (2020-2023) | Obra Social La Caixa, Health Research Call for Proposals | Elena Martínez |
| FINISHED PROJECTS | FINANCER | PI |
|---|---|---|
| ENGUT · Engineered models of intestinal epithelial tissue: assessing in vivo-like functional properties (2019-2020) | Bist Ignite Program | Elena Martínez |
| INDUCT Dispositivo de multitejido intestinal para la monitorización de la comunicación entre epitelio y músculo en condiciones patológicas (2018-2020) | MINECO | Elena Martínez |
| COMIET Engineering Complex Intestinal Epithelial Tissue Models (2015-2020) | ERC Consolidator Grant | Elena Martínez |
| GLAM Glass-Laser Multiplexed Biosensor (2015-2019) | European Commission (H2020) – PHC-10-2015 | Elena Martínez |
| MINAHE5 (Bio)funcionalización de Micro- y NanoHerramientas en Suspensión para Aplicaciones en Células Vivas (2015-2017) | MINECO | Maria Lluïsa Pérez |
Publications
Check for more detailed information on the outputs of the Group at IBEC CRIS portal.
Publications list:
Equipment
Micro and nanofabrication techniques:
- Biomolecule gradients produced by microfluidics
- Large-area nanostructured polymer surfaces produced by diblock copolymers
- 3D microstructures on hydrogel materials
- Mini-bioreactor for 3D cell culture
Characterization techniques:
- Surface Plasmon Resonance (SPR) measurements on polymer materials
- Atomic Force Microscope (AFM) expertise
- Optical Microscopes (white light/epifluorescence)
- Focused Ion Beam (FIB) / Scanning Electron Microscopy (SEM) of biological specimens
Equipment:
- Biological safety cabinet (class II)
- High precision syringe pumps
- Peristaltic pumps
- Access to the Nanotechnology Platform (IBEC Core Facilities): equipment for hot embossing lithography, polymer processing and photolithography, chemical wet etching, e-beam evaporation and surface characterization (TOF-SIMS)
- Access to the Scientific and Technological Centers (University of Barcelona): equipment for surface analysis (XPS, AFM, XRD) and microscopy techniques (SEM, TEM, confocal)
- PRUSA i3MK3S (FDM printer)
- Phrozen Shuffle XL (DLP-SLA printer)
- SOLUS (DLP-SLA printer)
Collaborations
- Prof. Ángel Raya / Dr. Samuel Ojosnegros
Center of Regenerative Medicine in Barcelona (CMRB), Barcelona - Prof. Eduard Batlle
Institut de Recerca Biomédica (IRB), Barcelona - Prof. Pablo Loza
Institut de Ciències Fotòniques (ICFO), Castelldefels (Spain) - Dr. Javier Ramón, IBEC
- Dr. Elisabeth Engel, IBEC
- Prof. Raimon Jané, IBEC
- Prof. Josep Samitier, IBEC
- Prof. Javier Santos, Dra. Maria Vicario
VHIR, Barcelona (Spain) - Dr. Bruno Sarmento
i3S – Instituto de Investigação e Inovação em Saúde, Porto, Portugal - Dr. Sonia García-Blanco
University of Twente, Enschede (The Netherlands) - Dr. Fabio Variola
University of Ottawa (Canada) - Dr. Daniel Riveline
ISIS/IGBMC, Strasbourg (France) - Dr. Matthew Dalby
University of Glasgow (UK) - Prof. Jordi Martorell
Institut de Ciències Fotòniques (ICFO), Castelldefels (Spain) - Prof. José Antonio Plaza
CNM-CSIC, Barcelona - Dr. Francesc Mitjans
LEITAT, Barcelona - Francesco Pampaloni
Buchmann Institute for Molecular Life Sciences (Frankfurt) - Dra. Elena Élez
VHIO
News

Bioengineering for precision medicine at the 18th IBEC Symposium
The 18th annual IBEC Symposium focused on ‘Bioengineering for Precision Medicine’, which is one of IBEC’s key areas of application. The event was attended by nearly 300 people, including local and international researchers. The multidisciplinary environment provided experts from other centres and the IBEC community with the opportunity to present their projects and exchange knowledge.

Seven additional IBEC labs achieve top-level in My Green Lab certification
Seven more research groups at the Institute for Bioengineering of Catalonia (IBEC) have been certified by My Green Lab, reaching the highest rating, the Green Level, for sustainable laboratory practices. With these additions, IBEC core facilities and 70% of the Institute’s laboratories are now certified.

IBEC and the Hospital del Mar formalise a new collaboration
The first collaboration day between the Institute for Bioengineering of Catalonia (IBEC) and the Hospital del Mar Research Institute was held today at the Barcelona Biomedical Research Park (PRBB). The meeting provided an opportunity to share research projects, identify areas for collaboration and formalise a strategic alliance through the signing of a collaboration agreement between IBEC, the Hospital del Mar and the Hospital del Mar Research Institute.
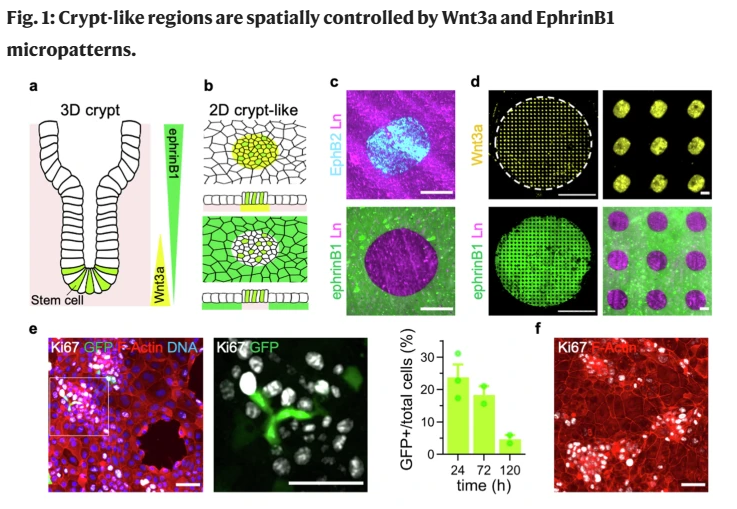
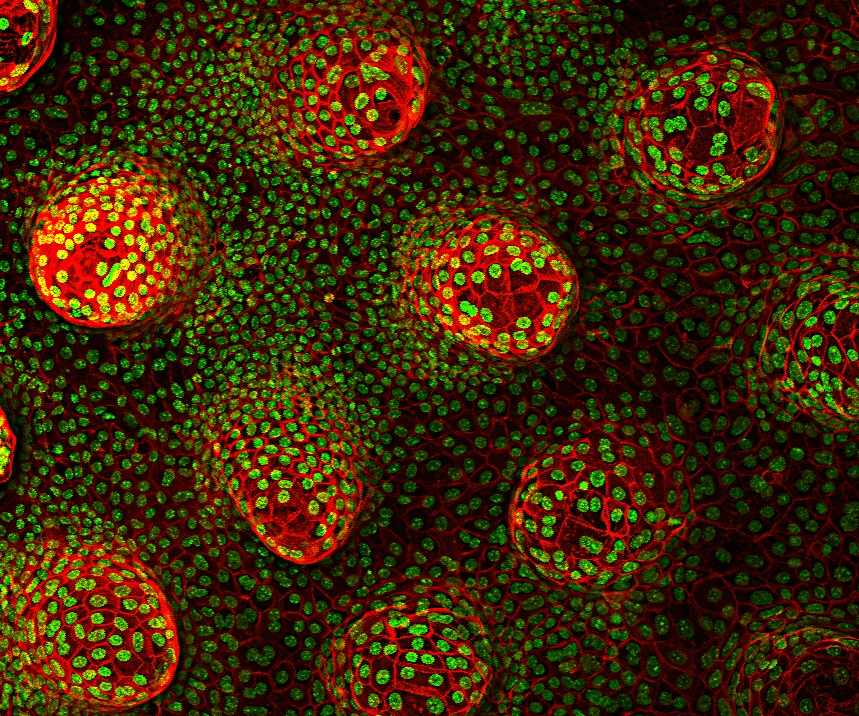
Madri+d: Impresión de proteínas para recrear en el laboratorio
Investigadores del grupo de Sistemas Biomiméticos para Ingeniería Celular del IBEC han desarrollado un método innovador para controlar la formación de criptas y vellosidades intestinales mediante impresión de proteínas por … Read more

Printing proteins to recreate the gut in the lab
An IBEC-led study describes the development of an innovative method to control the formation of crypt-like structures and villi in the intestine using a contact protein printing technique. This model will make it possible to study in detail key processes such as cell regeneration or changes associated with diseases such as cancer and chronic inflammatory disorders.

IBEC and VHIR hold a collaboration day to promote synergies
The 1st Translational Collaboration Day between the Vall d’Hebron Institute of Research (VHIR) and the Institute of Bioengineering of Catalonia (IBEC), held on 21st November, was an opportunity to learn about the projects and research lines of both institutions and to promote interaction between professionals.

IBEC and BST strengthen ties with Translational Collaboration Day
IBEC and the Blood and Tissue Bank of Catalonia (BST) held a day to explore new collaborations in bioengineering and translational medicine. The meeting, held yesterday at IBEC, highlighted innovative projects, presented a joint PhD programme and strengthened the link between biomedical research and clinical applications.

Bioengineering for Emergent and Advanced Therapies at the 17th IBEC Symposium
IBEC’s 17th Annual Symposium focused on ‘Bioengineering for Emergent and Advanced Therapies’, one of IBEC’s key application areas. Around 300 people attended the event, including local and international researchers. It was a multidisciplinary environment in which experts from other centres and the IBEC community itself had the opportunity to present their projects and share knowledge.

IBEC and West China Hospital strengthen collaboration in precision medicine
The second IBEC-WCH Precision Medicine Conference took place last week in Chengdu, China. This is a partnership between the Institute for Bioengineering of Catalonia (IBEC) and the West China Hospital (WCH) of Sichuan University, which aims to strengthen scientific collaboration between the two countries.

New bioink to mimic human skin constructs using 3D bioprinting
The demand for engineered skin tissues has surged for both in vitro and in vivo applications, and one of the key points to succeed is to choose an appropriate scaffold … Read more
Jobs
Predoctoral researcher at the Biomimetic systems for cell engineering Research Group (Project BIOCHIC)
Ref: FPI_EM // Deadline: 29/10/2025
Scientific business developer at the Biomimetic systems for cell engineering Research Group
Ref: BD-EM // Deadline: 03/10/2025
Laboratory Technician at the at the Nanobioengineering Group
Ref: LT-EM // Deadline: 4/7/2025
Research Assistant at the Biomimetic systems for cell engineering Research Group
Ref: RA-EM // Deadline: 21/06/2025
Laboratory Assistant at the Biomimetic systems for cell engineering Research Group
Ref: LA-EM // Deadline: 20/06/2025
Research Assistant at the Biomimetic Systems for Cell Engineering Research Group
Ref: RA-EM //Deadline: 25/02/2025
Senior researcher at the Biomimetic Systems for Cell Engineering Research Group (Ref. SRR_EM)
Ref: SRR_EM // Deadline: 31/10/2023
Research Assistant at the Biomimetic systems for cell engineering Research Group (RA_EM)
Ref: RA_EM // Deadline; 07/08/2023
Postdoc at the Biomimetic systems for cell engineering Research Group (PD_EM)
Ref: PD_EM // Deadline: 04/08/2023
Laboratory technician at the Biomimetic Systems for Cell Engineering Research Group (LT_EM)
Ref: LT_EM // Deadline 10/02/2023


 ibecbarcelona.eu
ibecbarcelona.eu