ABOUT
Our lab’s focus is on understanding the mechanisms that control mammalian embryo implantation and apply that knowledge to provide solutions that improve human assisted reproduction techniques (ART).
The embryonic development of humans (and mammals in general) requires the implantation of the embryo into the walls of the maternal uterus. This process is highly inefficient as on average, 25–30% of conceptions lead to successful live births and around 60% of all conceptions are lost at the time of (or soon after) implantation. However, despite the central role of implantation in human fertility, the process is still elusive to experimentation because of its inaccessibility.
To overcome the elusiveness of this process, the group combines imaging and bioengineering methods to efficiently culture and image pre-implantation embryos and allow them to implant outside the uterus in highly physiological conditions. Our systems are accessible to imaging tools which allow us to interrogate the genetics, metabolomics, and mechanics of the embryo in a high throughput manner. Using our systems, we are capable to (i) improve embryo culture conditions and (ii) diagnose embryos with improved implantation potential.
Label-free microscopy and multi/hyperspectral imaging
Label-free Microscopy: This technique allows for analyzing cells in their native condition, i.e. without being labeled or altered in any way, by means of retrieving cells autofluorescence signals and thus providing essential metabolic information about living tissues. Combined with multispectral methods, we delve deep into the metabolic complexity of embryos and oocytes, revealing insights previously unattainable.
Hyperspectral Imaging: Our groundbreaking approach employs hyperspectral imaging to obtain the metabolic profiles of embryos and oocytes. This methodology allows us to identify key characteristics at the metabolic level, invisible to conventional techniques like brightfield imaging, offering a unique window into fundamental biological processes.
Hardware Techniques: We have extensive expertise in a variety of advanced microscopy techniques, including two-photon microscopy, laser scanning confocal microscopy, spinning disk confocal microscopy and light-sheet microscopy. The integration of these techniques with multi/hyperspectral detection methods enables us to observe biological samples of interest at cellular level with spectral characteristics.
AI and Software Analysis: We employ sophisticated data analysis tools such as spectral histogram analysis and phasor-plot analysis combined with artificial intelligence (AI) methods for classification. These techniques allow us to interpret complex multi/hyperspectral data and draw meaningful conclusions about the viability and quality of embryos and oocytes.
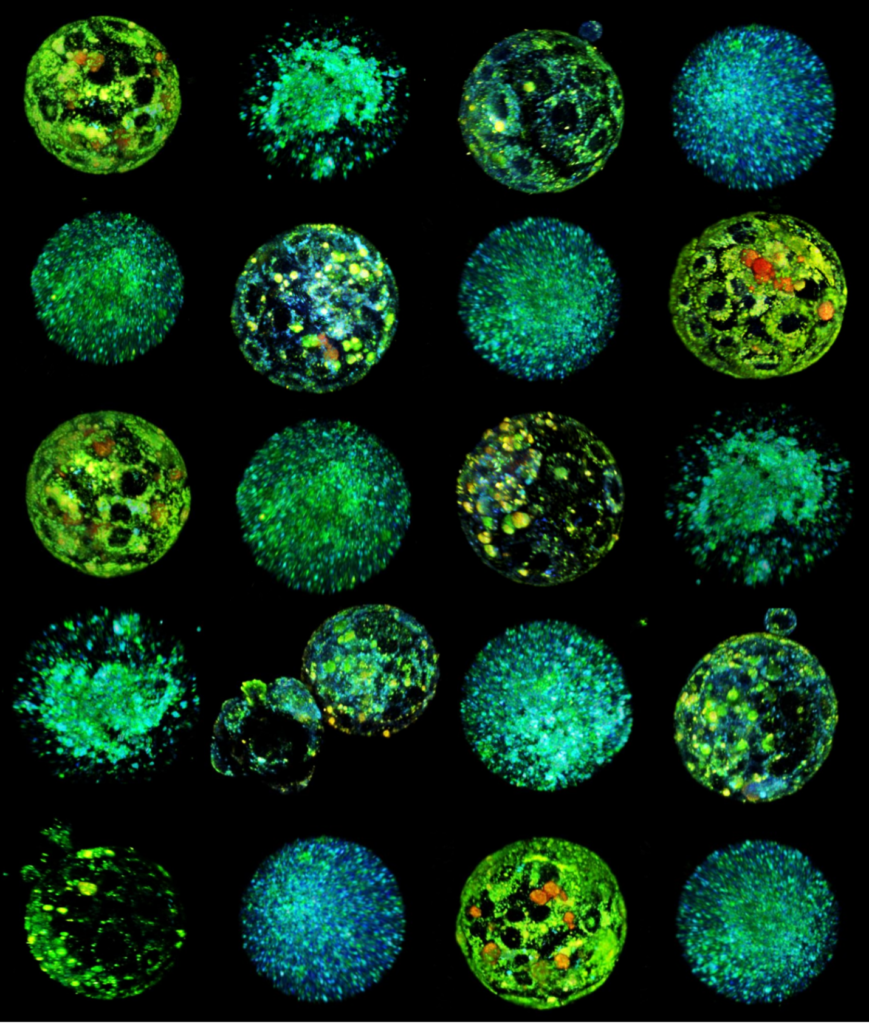
4D (x, y, z, and λ) Hyperspectral Autofluorescent Images of Mouse Oocytes and Blastocysts. The samples were imaged using a Zeiss LSM780 inverted microscope with a C-Apochromat 40x/1.20 W Korr Zeiss objective. The imaging conditions were maintained at 37°C and 5% CO2. A Mai-Tai DeepSee laser provided two-photon excitation at a wavelength λex = 780nm. The emitted autofluorescence spanned from 410 nm to 695 nm and was collected by a 32-channel PMT GaAsP spectral detector. The image captures multiple planes, separated by 2.5μm and 5μm steps for oocytes and embryos respectivelly. The pseudo-color representation of the hyperspectral images was achieved using additive blending to integrate the 32 channel colors for each pixel.
Bioengineering to improve embryo implantation
We have developed proprietary 3D ex vivo hydrogel-based implantation platforms which mimic the uterine microenvironment, allowing the embryo to progress towards post-implantation stages in an amenable way for optical microscopy. Working towards obtaining dynamic control of embryo culture, we have integrated our hydrogels in a microfluidic device allowing for controlled nutrient supply, oxygen concentration and long-term embryo culture. Our 3D ex vivo hydrogel-based implantation platforms allow for drug screening and determination of its impact on embryo implantation and development.
We use our 3D ex vivo implantation platform to understand embryo implantation with a focus on the biomechanics of the system. To this means we quantify the displacement of the matrix generated by the embryos using PIV or DVC algorithms. We look at the forces and resulting patterns embryos are applying in order to penetrate the hydrogel and also how external forces affect embryo implantation.
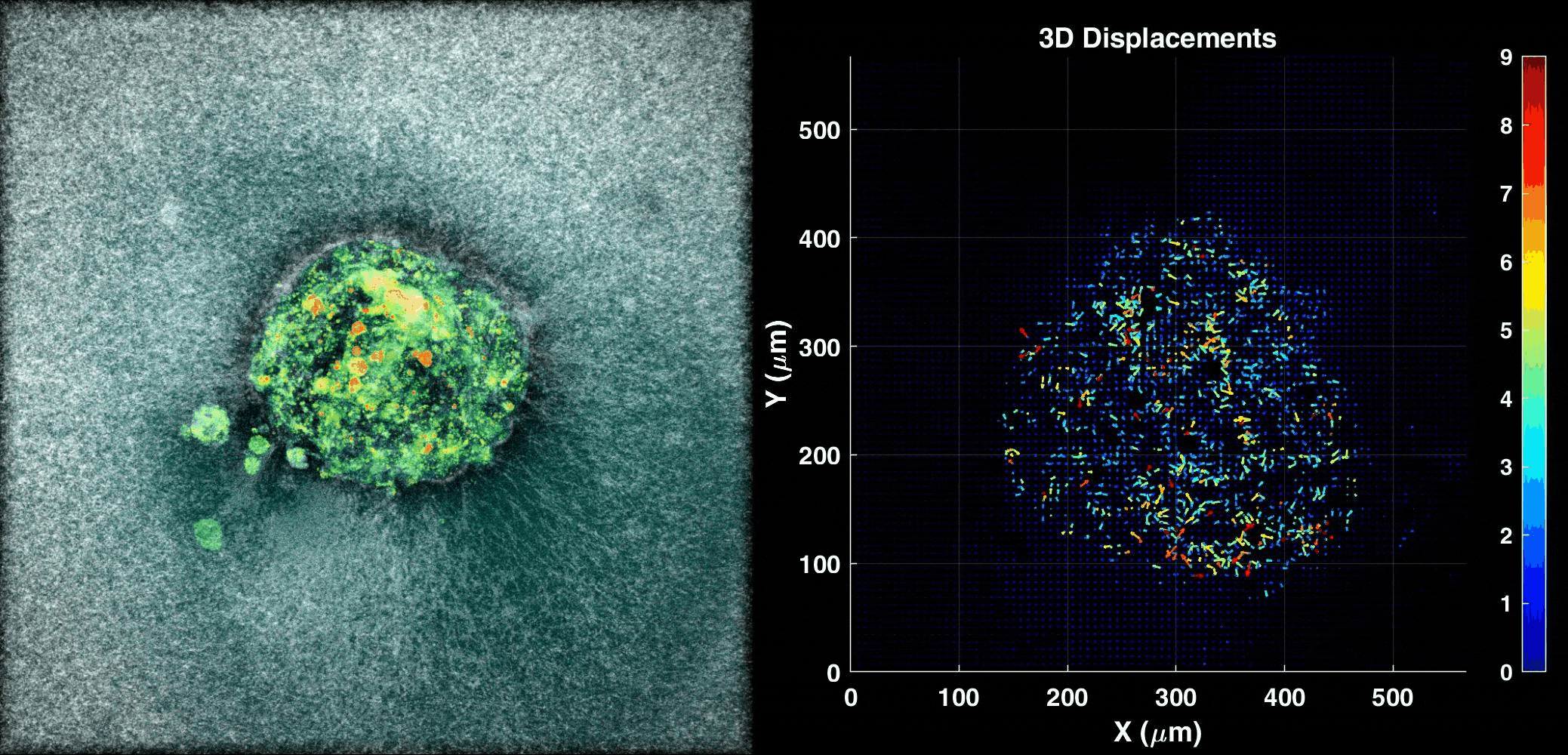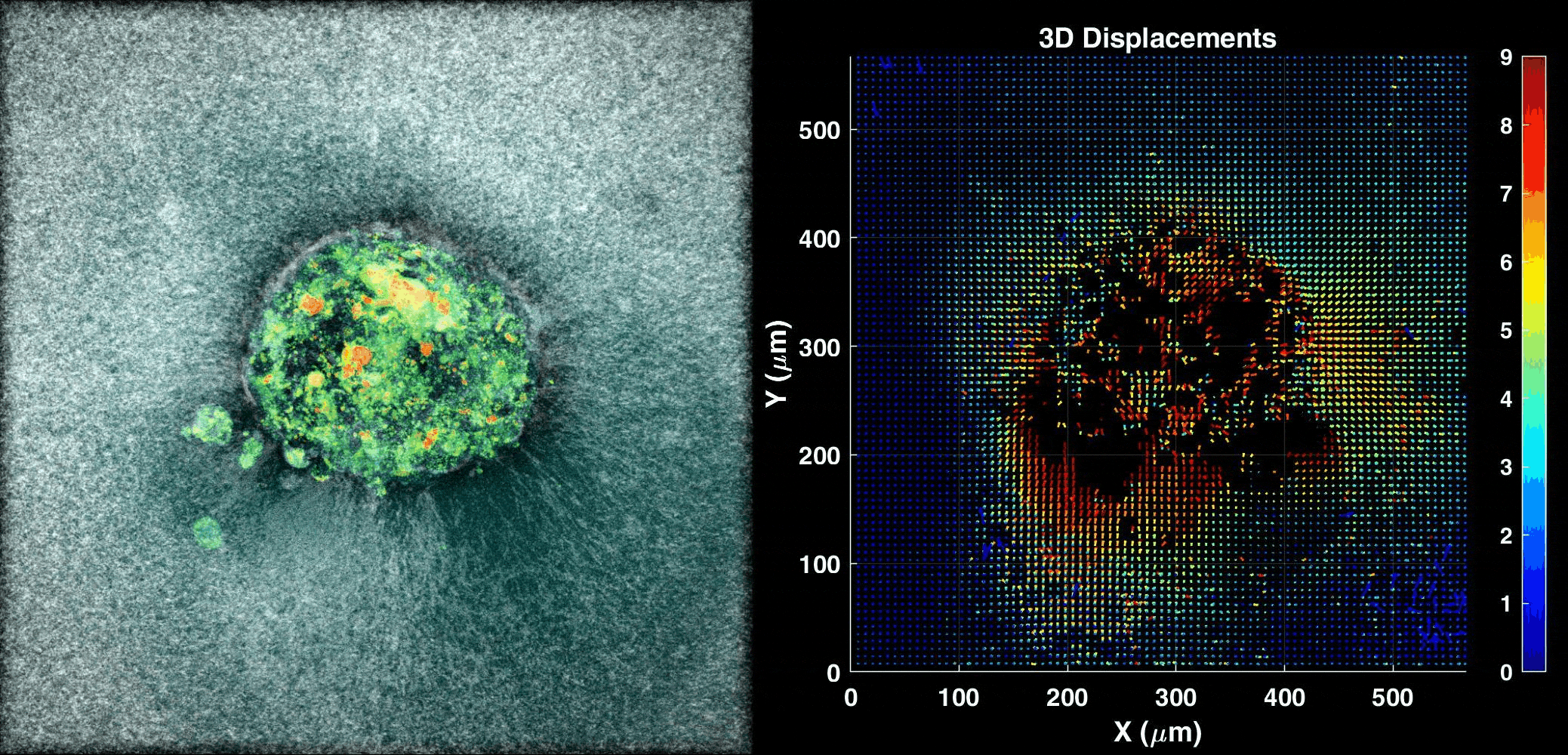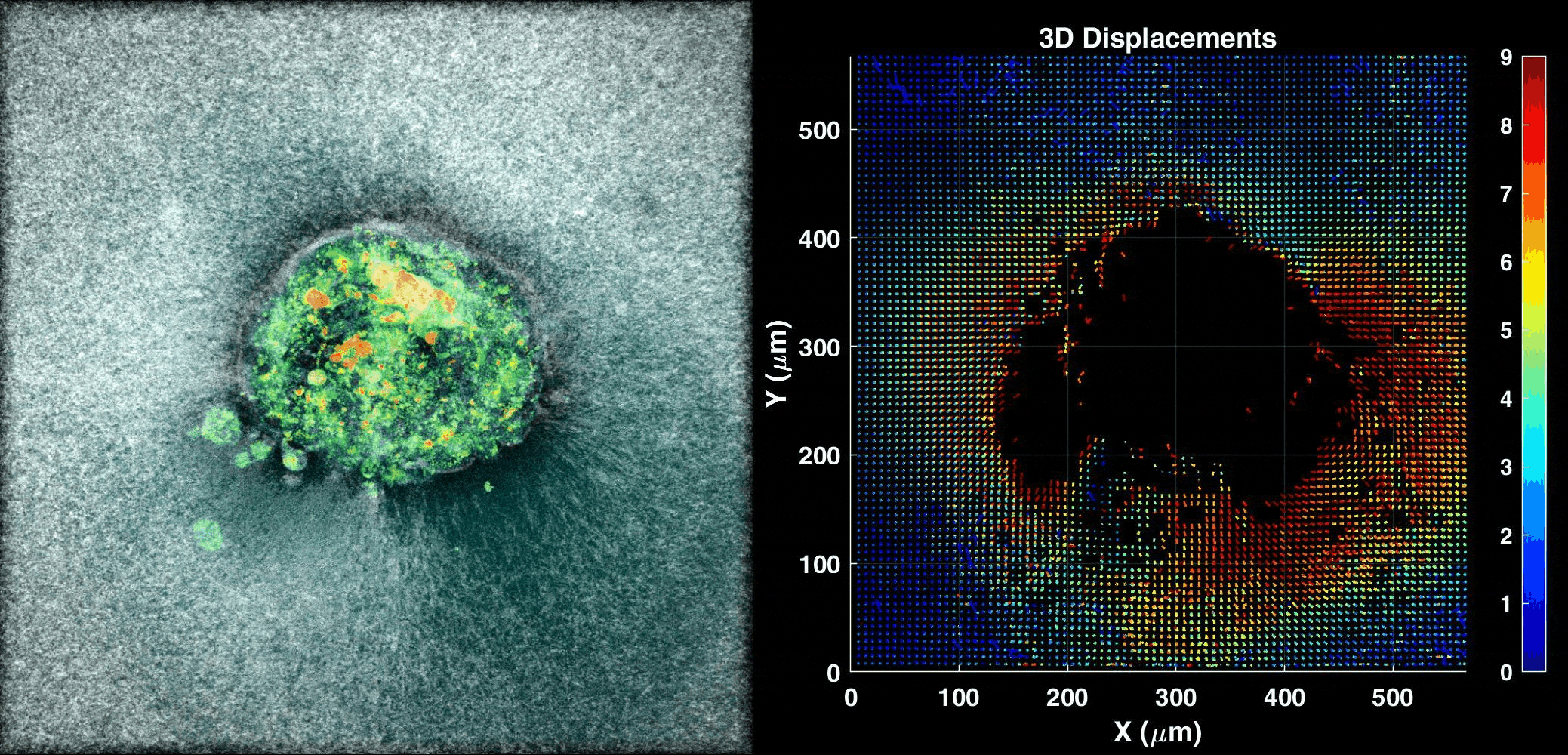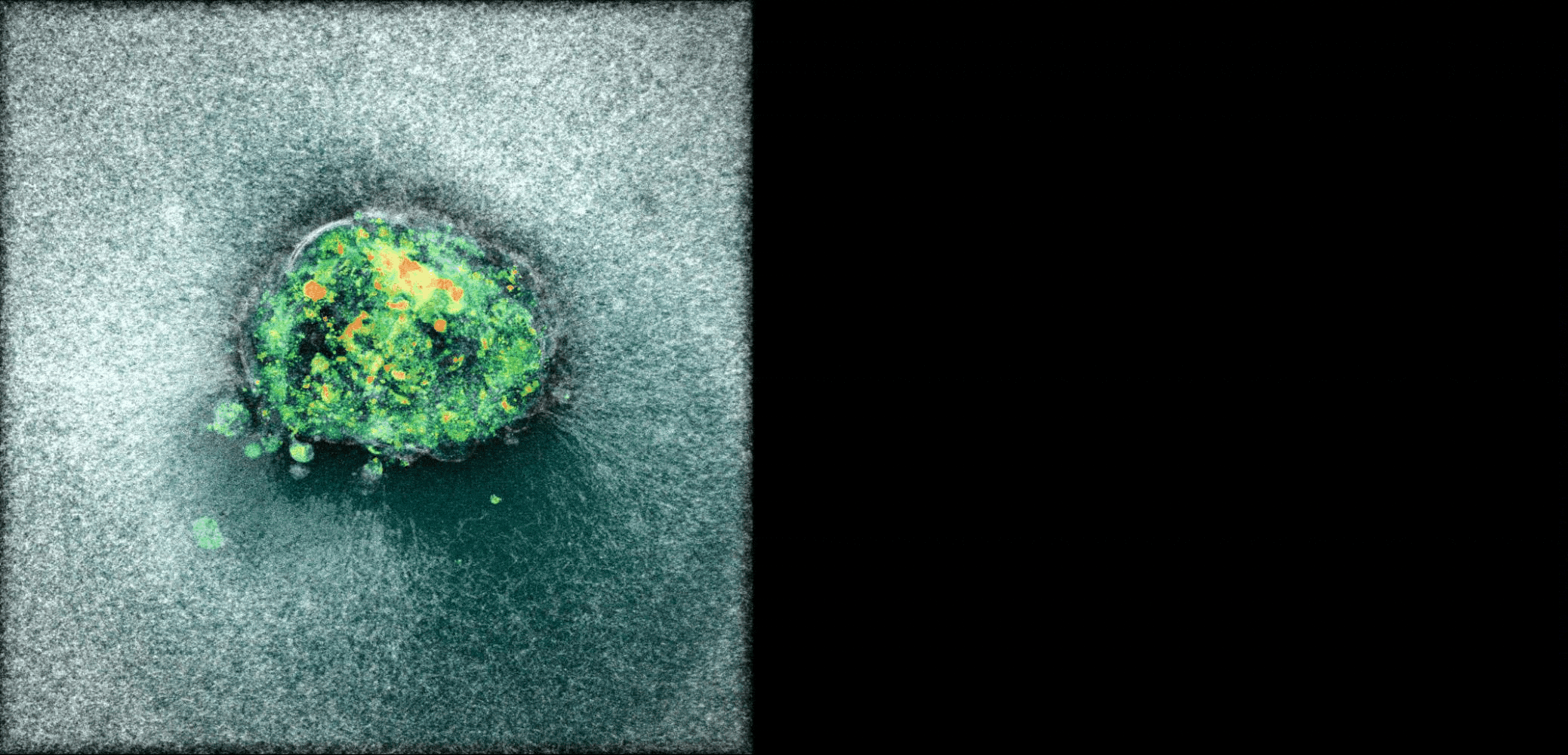
Human Embryo implanting on a 3D platform
Embryo Culture Supplements Development from Human Plasma
Traditionally, embryo culture relied on Human Serum Albumin (HSA) as a key protein component. However, HSA underrepresents the rich composition of proteins present in human plasma. We work with a new-generation of supplements, which go beyond mere albumin, encompassing essential components such as growth factors and globulins, crucial for fostering optimal embryo development. In our group, we test new supplements to enhance embryo development and implantation. Our clinical grade human-derived supplements improve blastulation and implantation rates both in human and mouse embryos, showing superior lineage segregation and spatial organization compared to control counterparts.
The Bioengineering in Reproductive Health is the first Open Innovation Lab research unit at IBEC.
Due to the high translational component of our research, we have established collaboration contracts with the pharma industry, hospitals, and venture capital to bring our technology to the clinics and the market. Our Open Lab is a multidisciplinary environment where embryologists, cell biologists, optical physicists, biophysicists, and business developers synergize to create a unique environment shaped by science and entrepreneurship.
STAFF
PROJECTS
European Projects
| HSMe-ImPredict · Development of non-invasive imaging methodology for improving embryo implantation prediction, via hyper-spectral metabolic profiling (2022-2024) | Marie Curie Individual Fellowship | Samuel Ojosnegros |
National projects
| HYSPLANT · Selección de embriones para fecundación in vitro: predicción del éxito de implantación mediante clasificación metabólica de embriones (2020-2023) | MINECO Retos investigación: Proyectos I+D | Samuel Ojosnegros |
| Prediction of implantation success by hyperspectral metabolic profiling of human embryos obtained by in vitro fertilization (2020-2022) | AGAUR Beatriu de Pinós 2018 | Samuel Ojosnegros |
Private sector
| Estudio del mecanismo de acción de OXO-001 (2020-2022) | Oxolife | Samuel Ojosnegros |
| Evaluación de derivados plasmáticos humanos para el cultivo de embriones (2019-2021) | IVFTECH S.L. | Samuel Ojosnegros |
| Evaluación de derivados plasmáticos humanos para el cultivo de células mesenquimales y CAR-T (2020-2022) | IVFTECH S.L. | Samuel Ojosnegros |
| Prediction of implantation success by single-cell transcriptomic profiling of human embryos obtained by in vitro fertilization (2018-2022) | Scranton Enterprises BV | Samuel Ojosnegros |
Finished projects
| HYSPLANT · Metabolic profiling of in vitro fertilization embryos using hyspectral imaging (2019-2020) | European Commission ATTRACT | Samuel Ojosnegros |
| Embryo on a chip: Smart microdevice development for high-throughput screening embryo implantation (2020) | Tecniospring 2020 | Maria Demestre Viladevall |
NEWS

LUMIRIS, an IBEC spin-off, wins the Digital Entrepreneur Award in the Revelation category
The 7th edition of the Premis Emprenedors Digitals (Digital Entrepreneur Awards), organised by the Catalunya Ràdio programme, took place on Wednesday 18 June at the Antigua Fábrica Damm in Barcelona. The award recognises companies that have demonstrated exceptional growth and innovation in their field.
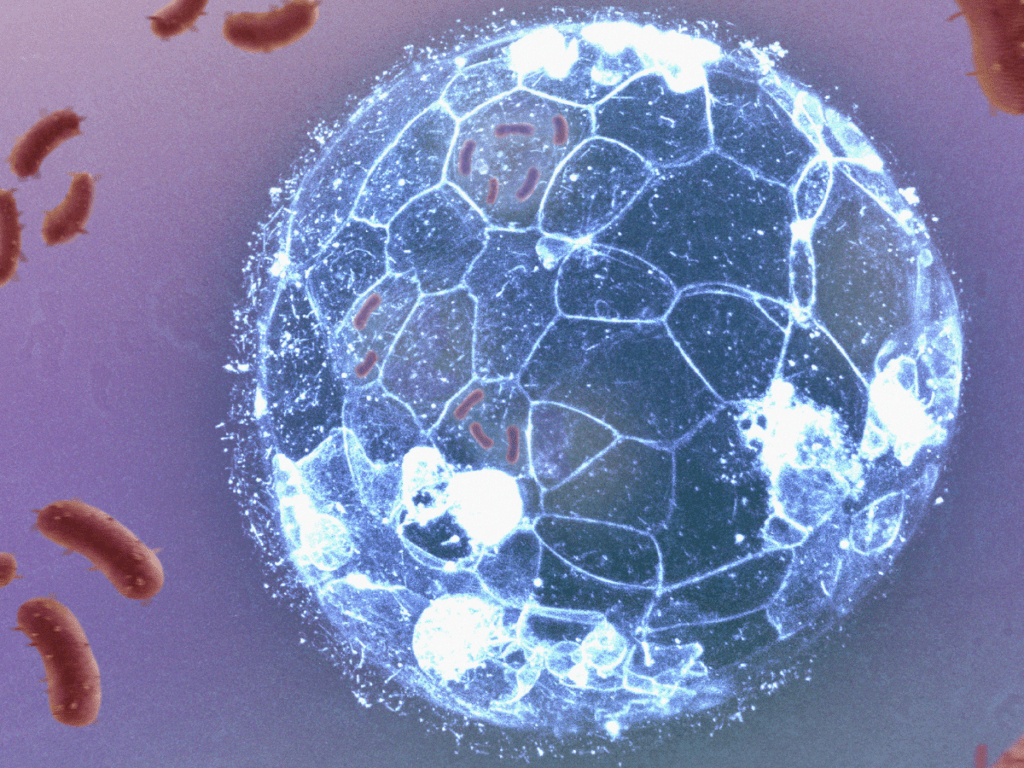
Embryos can eliminate bacterial infections before forming their immune system, a new research shows
The work, led by a team from the CSIC and IDIBELL, with the collaboration of IBEC, manages to visualise how embryonic cells eliminate bacterial infections, before the formation of the immune system. The research describes a mechanism of phagocytosis similar to that used by white blood cells, and reveals that this mechanism is also present in human embryos.

Printing proteins to recreate the gut in the lab
An IBEC-led study describes the development of an innovative method to control the formation of crypt-like structures and villi in the intestine using a contact protein printing technique. This model will make it possible to study in detail key processes such as cell regeneration or changes associated with diseases such as cancer and chronic inflammatory disorders.

Catalunya Radio: Lumiris: l’empresa que transforma la reproducció assistida
Lumiris es una empresa derivada del Instituto de Bioingeniería de Cataluña que ha desarrollado una tecnología no invasiva para analizar el metabolismo de embriones humanos, permitiendo seleccionar los más aptos … Read more

El Español: La tecnología no invasiva que revoluciona la reproducción asistida: Lumiris avanza a paso firme hacia el mercado
El diagnóstico por imagen del IBEC reduce el tiempo y los ciclos de tratamiento de la fecundación in vitro. Su última ronda eleva a seis millones la financiación lograda para … Read more

LUMIRIS, the IBEC spin-off that has raised €6 million to transform assisted reproduction
This spin-off from the Institute for Bioengineering of Catalonia has developed a non-invasive technology that analyses the metabolism of human embryos to select the most suitable ones for implantation. This innovation promises to significantly reduce the time and number of treatment cycles required for in vitro fertilisation. The success of LUMIRIS’ technology has led to a new round of investment of €1.5 million, bringing the total to €6 million since the company was founded in 2023.

IBEC and VHIR hold a collaboration day to promote synergies
The 1st Translational Collaboration Day between the Vall d’Hebron Institute of Research (VHIR) and the Institute of Bioengineering of Catalonia (IBEC), held on 21st November, was an opportunity to learn about the projects and research lines of both institutions and to promote interaction between professionals.

IBEC and BST strengthen ties with Translational Collaboration Day
IBEC and the Blood and Tissue Bank of Catalonia (BST) held a day to explore new collaborations in bioengineering and translational medicine. The meeting, held yesterday at IBEC, highlighted innovative projects, presented a joint PhD programme and strengthened the link between biomedical research and clinical applications.
JOBS
Scientific Liaison at the Bioengineering in Reproductive Health Research Group
Ref: SL-SO // Deadline: 16/06/2025
Postdoctoral Researcher at the Bioengineering in Reproductive Health Research Group/ Unit
Ref: PR_SO // Deadline: 13/11/2024
Embryology Laboratory Technician at the Bioengineering for Reproductive Health Research Group
Ref: PD-SO/Deadline: 04/04/2024
Open postdoc position on embryology at the Bioengineering in Reproductive Health Research Group
Ref: PD_SO/ Deadline: 12/02/2024
Research Assistant at the Bioengineering for Reproductive Health Research Group
Ref: RA_SO/Deadline: 20/11/2023
Postdoc on microscopy at the Bioengineering in Reproductive Health Research Group
Ref: PD_SO//Deadline: 1/11/2023
Postdoctoral researcher at the Bioengineering in Reproductive Health Open Lab (Ref: PD_SO)
Ref: PD_SO // Deadline: 19/01/2023
Embryologist at the Bioengineering in Reproductive Health Research Group (Ref: LT_SO)
Ref: LT_SO // Deadline: 19/01/2023
Biochemist (with expereince in hematology) at the Bioengineering in Reproductive Health Research Group
Reference: LT-SO / Until November 19th, 2022
PUBLICATIONS
S Ojosnegros, A Seriola, AL Godeau, A Veiga (2021) Embryo implantation in the laboratory: an update on current techniques. Human Reproduction Update, Vol.00, No.0, pp. 1–30.
Martin Plöschner, Denitza Denkova, Simone De Camillis, Minakshi Das, Lindsay M. Parker, Xianlin Zheng, Yiqing Lu, Samuel Ojosnegros, and James A. Piper (2020) Simultaneous super-linear excitation-emission and emission depletion allows imaging of upconversion nanoparticles with higher sub-diffraction resolution. Optics Express 28 (16), 24308-24326.
EQUIPMENT
- Embryo culture laboratory
- IFV workstations in laminar flow hoods
- Microscope
- Micromanipulation-microinjection station
- Embryo biopsy laser
- K-systems incubator
- Cell culture laboratory
- Biosafety cabinets
- Incubators
- Automated cell counter
- Dry warming/thawing system
- Sterile tubing welder
- Tubing sealer
- Centrifuges
- Advanced live imaging: photoconversion, 3D imaging, light scattering, spectroscopy
- Crest spinning disk mounted on a Nikon Ti
- Image analysis workstation
COLLABORATIONS
- Prof. Anna Veiga – Barcelona Stem Cell Bank (IDIBELL) and Dexeus Mujer, Barcelona
- Dr. Montserrat Boada/ Dr. Pere Barri – Dexeus Mujer, Barcelona
- Dr. Ayelet Lesman – Tel Aviv University (TAU), Israel
- Dr. Elena Martínez – IBEC
- Dr. Francesco Cutrale, University of Southern California (USC), USA
- Dr. Manuel Irimia – CRG, Barcelona
- Dr. Javier Ramón – IBEC
ENTREPRENEURSHIP
· Jorge Fuentes,
Business Strategy, A_Ventures, Barcelona, Spain
 |  |  | |
 |  |
Follow us on Twitter: @Biorephealth



