About
Cells continuously sense their environment by integrating multiple input signals from neighbouring cells and respond to them with a fast-flowing cascade of abundant outputs. In turn, these outputs modify the environment.
Taking a set of distinct cell types, this spatiotemporal combinatorial phenomenon can presumedly lead to a vast number of multicellular spatial patterns (Figure 1). Still, only a relatively limited number of stable multicellular spatial patterns with functions (and dysfunctions) emerge from all possible coordination events amongst multiple cell types. For these patterns to repeatedly arise in distinct organisms, a set of principles driving the spatial organization of multiple cell types must be conserved; however, these principles remain largely unknown as we have only recently begun to understand how multicellular organization works. Being able to read, anticipate the outputs, and eventually control the principles behind the spatial organization of multiple cell types are, to us, some of the most fascinating problems of modern biotechnology. Advances in this area are expected to be key to manipulate tissue function and, therefore, they will undoubtedly bring numerous benefits to society: from a deeper understanding of the natural world to better cell-based therapies, diagnostics tools, and engineered tissues for precision and regenerative medicine.
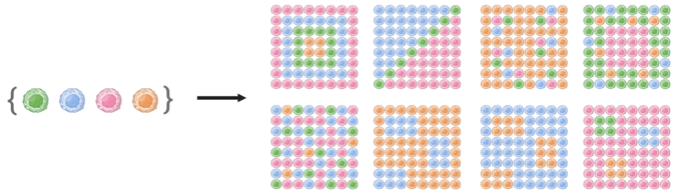
Figure 1. A set of cell types can theoretically arrange in a vast number of spatial patterns.
In the Spatial Biotechnology group, we are making headway on this problem via the in-depth study of clonal behaviors in solid tumors. The number of cancer patients is expected to skyrocket in the next few decades in part due to the progressive aging of the population. Therefore, it is imperative to improve and expedite cancer management. A common reason for the failure of current therapies in solid tumors is that they are highly heterogeneous entities, and a major source fueling this heterogeneity is clonal diversity, which encompasses genetic variability and epigenetically regulated cell states. Clonal diversity significantly influences tumor growth, metastasis, and therapy response. It is increasingly clear that the spatial organization of cancer cell clones is a crucial factor in solid tumor development, as cell fate is intimately linked to the local environment. However, our understanding of the rules governing clonal spatial organization in tumors is limited, largely due to the absence of mature experimental and computational frameworks to investigate clonal behaviors in the native tissue context.
The goal of the Spatial Biotechnology group is to develop high-throughput and spatial perturbation technologies to understand how cellular clones spatially organize to drive solid tumor development, with the long-term goal of designing better cancer therapies and tools to predict clinical outcomes.
To achieve this goal, our group develops experimental and computational approaches for spatial multi-omics, and it applies them to interrogate and engineer a variety of clonal behaviors in solid tumors from clinical sources, murine models, and in vitro tissues. This interdisciplinary work builds on our expertise in developing highly multiplexed tissue imaging technologies, super-resolution, mass spectrometry, and multimodal microscopy, pooled genetic screens, assay automation strategies, immune cell engineering approaches, and computational tools for deconstructing molecular and cellular spatial patterns in cells and tissues (Figure 2).
In the Spatial Biotechnology group, we focus on some of the most pressing questions in the field, specifically:
1) How is clonal cooperation established and maintained?
The widespread clonal heterogeneity of human tumors implies that several ecological interactions are in play, together with clonal competition. Indeed, recent evidence supports that tumor evolution could be also driven by clonal cooperation, where growth equilibrium of two or more clones might be a path to optimal fitness. Using genome editing tools, pooled screens, and super-resolution multiplexed imaging, we interrogate molecular signatures of tens of genetically engineered cancer cell clones in the native tissue context in in vivo murine models. This work aims to characterize molecular determinants promoting clonal cooperation in cancer, which would provide causal understanding on how genetically unique clones arrange within a tumor and regulate tumor growth.
2) How does metabolite accessibility regulate the growth of neighboring cancer cell clones?
Distinct environmental conditions simultaneously arise in multiple regions of a tumor providing selective pressure to the variable cancer cell population at place, further increasing heterogeneity or dramatically shifting the composition of the clonal pool. For instance, metabolic reprograming of cancer cells can result in localized and differential enrichment of metabolites. Using automation strategies and highly multiplexed imaging, we characterize clonal cancer cell behaviors in hundreds of in vitro 3D tumors growing in metabolically defined environmental conditions. This work aims to identify clonal signatures in specific metabolic environments, which would provide clues on how the environment regulates clonal growth and potentially inform future therapeutic interventions.
3) How do clonal populations and microenvironments evolve in primary tumors?
The tumor microenvironment, which includes resident immune and stromal cells as well as the extracellular matrix, is closely linked to the evolution of the cancer cell population. Using spatial transcriptomics and histopathology-grade proteomics, we correlate molecular features that are predictive of clonal growth in a variety of patient cohorts in collaboration with clinicians. This work aims to identify clonal patterns in clinical tumor samples, which would inform about spatial features correlating with clinical outcome for better disease management.
4) How could adoptive T-cell transfer therapies be improved for solid tumor treatment?
Some of the major obstacles to increase the success of adoptive T-cell transfer therapies for solid tumors is limited T-cell infiltration, persistence, and functionality. Using immune cell engineering approaches and highly multiplexed imaging, we track the tumor location of adoptive T-cell transfer therapies and assess their functional status in the native tissue environment of models permissive to T-cell infiltration. This work aims to identify molecular determinants of productive T-cell fitness, which would provide alternative options to improve current cell-based therapies for cancer.
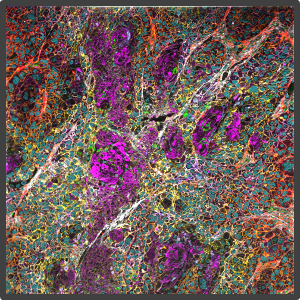
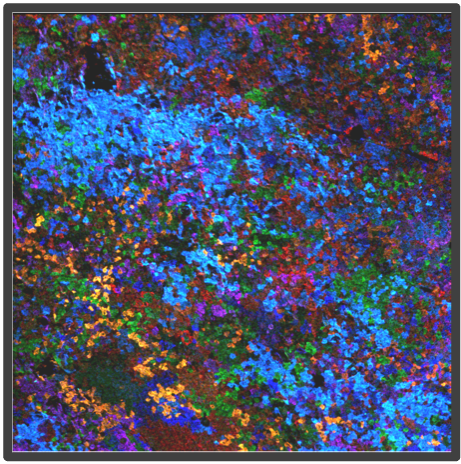
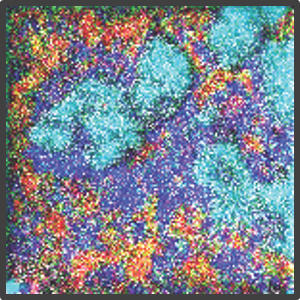
Figure 2. Representative highly multiplex images in a variety of models analyzed by Multiplex Ion Beam Imaging (MIBI), a spatial proteomics technology that detects 40+ antibodies in tissue sections using isotopes. (Left) Histopathology-grade MIBI in a section of human squamous cell carcinoma. (Middle) Clonal tracing studies using MIBI in a tumor grown in vivo. (Right) Super-resolution multiplex imaging of the nucleus of a cisplatin-treated TYK-nu cell, an ovarian cancer cell line.
Staff
Xavier Rovira Clavé
Projects
Publications
Collaborations
- Prof. Garry Nolan
Department of Pathology, Stanford University, United States - Prof. Julien Sage
Department of Pediatrics, Stanford University, United States - Dr. Alexandros Drainas
BioMed X Institute, Heidelberg, Germany - Dr. David McIlwain
University of Nevada, Reno, United States - Dr. Sizun Jiang
Center for Virology and Vaccine Research, Harvard Medical School, United States - Dr. Franzi Blaeschke
German Cancer Research Center (DKFZ), Heidelberg, Germany - Dr. John Hickey
Department of Biomedical Engineering, Duke University, United States - Dr. Jolanda Sarno
Tettamanti Research Center, University of Milano-Bicocca, Italy - Prof. Miriam Cuatrecasas
IDIBAPS, Barcelona - Dr. Jordi Camps
IDIBAPS, Barcelona
News

New technology for mass serological analysis
A study by Stanford University and the Institute for Bioengineering of Catalonia describes an innovative technology that enables the large-scale analysis of antibodies in biological samples. Using microscopic beads marked with stable isotopes, this advance surpasses traditional techniques, accelerating the study of immune responses and opening up new possibilities for biomedical research.
IBEC receives funding from the prestigious Human Frontier Science Program
The Institute for Bioengineering of Catalonia (IBEC) is participating in the international SOLFEGE project, which aims to explore how different cell types coordinate with each other through soluble factors in the tumour microenvironment. This project has been made possible thanks to funding from the Human Frontier Science Program. SOLFEGE is a consortium led by the German Cancer Research Center (DKFZ), with IBEC and the Duke University, as partners.

IBEC and VHIR hold a collaboration day to promote synergies
The 1st Translational Collaboration Day between the Vall d’Hebron Institute of Research (VHIR) and the Institute of Bioengineering of Catalonia (IBEC), held on 21st November, was an opportunity to learn about the projects and research lines of both institutions and to promote interaction between professionals.

Advances in nanobiotechnology and nanomedicine explored at the 12th International NanoBio&Med Conference
The latest developments in nanotechnology, biotechnology and medicine were presented at the NanoBio&Med 2024 International Conference, held this week at the Barcelona Science Park (PCB) from 5 to 7 November. This is an annual event designed to establish new collaborations and promote innovative projects in the scientific-industrial sector.

IBEC and BST strengthen ties with Translational Collaboration Day
IBEC and the Blood and Tissue Bank of Catalonia (BST) held a day to explore new collaborations in bioengineering and translational medicine. The meeting, held yesterday at IBEC, highlighted innovative projects, presented a joint PhD programme and strengthened the link between biomedical research and clinical applications.

IBEC is strengthened by the addition of three new research groups in advanced and emerging therapies
IBEC kicks off 2024 with the incorporation of three new research groups led by Manuel Salmerón Sánchez, Zaida Álvarez Pinto, and Xavier Rovira Clavé. With these additions, IBEC strengthens its position in the field of advanced and emerging therapies.

The ERC supports the “SpaceClones” project to understand the clonal evolution of tumors
Xavier Rovira Clavé, currently a researcher at Stanford University’s School of Medicine, has been awarded the “Starting Grant” from the European Research Council (ERC) to carry out his project “SpaceClones” … Read more
Jobs
Predoctoral Researcher at the Spatial Biotechnology Research Group
Ref: PhD_XR // Deadline: 2/06/2025
Postdoctoral researcher at the Spatial Biotechnology Research Group
Ref: PR_XR//21/02/2025
Predoctoral Researcher at the Spatial Biotechnology Research Group
Ref: PhD-XR // Deadline: 28/08/2024
Predoctoral Researcher at the Spatial Biotechnology Research Group
Ref: PhD_XR//Deadline: 22/03/2024


 ibecbarcelona.eu
ibecbarcelona.eu