One of the great challenges in the fight against cancer is to design new technologies for a personalized treatment for each patient. Depending on the molecular characteristics —DNA mutations for instance— of each tumour, precision medicine aims to make it easier for cancer patients, both adult and paediatric, to receive a personalized treatment that is appropriate to their pathology. But is it possible to know whether or not a patient can benefit from a treatment before starting therapy?
A team of experts from the e Institute for Bioengineering of Catalonia (IBEC) and the Faculty of Medicine and Health Sciences of the University of Barcelona has designed a microfluidic device called microfluidic dynamic BH3 profiling (μDBP) that predicts the effectiveness of cancer treatment quickly and automatically, using a small number of cells from biopsies and without requiring specialised technical staff.
The study, published in the journal npj Precision Oncology, was led by Joan Montero, professor at the UB Department of Biomedicine and IBEC, and Javier Ramón-Azcón, ICREA research professor at IBEC. The paper, whose first author is Albert Manzano (UB-IBEC), who received his PhD from the UB in 2022 with a thesis on precision medicine in the fight against cancer, involves experts from the UB Faculty of Physics, the Vall d’Hebron Institute of Oncology (VHIO) and the Biomedical Research Networking Center in Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN).
Precision medicine in the fight against cancer
Personalized medicine has revolutionised the way we design effective cancer treatments. Considering that each tumour is unique and has its own characteristics, having predictive indicators of each patient’s response to treatment is a major step forward in oncology. The dynamic BH3 profiling (DBP) was initially developed in the laboratory of Professor Anthony Letai —Professor Montero was a co-inventor in this study— and patented in 2015 by the Dana-Farber Cancer Institute (United States). It was one of the first functional assays successfully tested to predict treatment in various types of cancer. This system brings cancer cells into contact with different therapeutic options to quickly identify ex vivo the ones that might be most effective in removing the tumour. Conceptually, it is very similar to the antibiograms used to identify antibiotics to treat bacterial infections.
“DBP has been used to identify the efficacy of treatments on a preclinical and clinical scale in many different cancers, both solid and liquid. These studies have used cell lines, animal models and primary samples with high predictive ability in all cases. However, this assay has not yet been widely applied in hospitals”, says lecturer Joan Montero.
“So far —he adds— several studies have found a good correlation between DBP results and clinical response in primary leukaemia samples. There are currently several clinical trials underway, and we would like this technology to be implemented in hospitals in the coming years to improve cancer therapies”.
Predicting the therapeutic response with few cancer cells
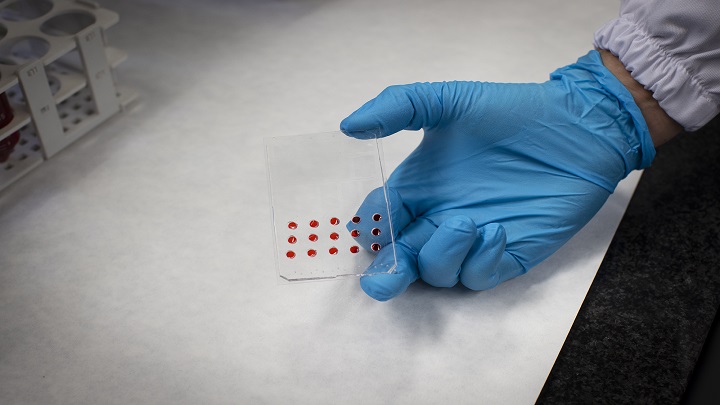
Now, the new DBP microfluidic device —known as μDBP— solves several functional assay challenges: it reduces the number of cancer cells needed to test potential ex vivo therapies and automates the process to facilitate clinical application without specialised technical staff.
“One of the main limitations of the DBP is the number of cells needed to perform the assay. When a biopsy is performed on a patient, the number of tumour cells we obtain is very limited, which does not allow a study with many different treatments and limits the ability to identify one that is effective”, notes Albert Manzano.
When a biopsy is received, the sample is dissociated to obtain individual cells using mechanical and enzymatic treatments. Once processed, the sample is filtered to obtain individual cells which are then subjected to the desired treatments and seeded in the microfluidic device.
“Thanks to our μDBP microfluidic platform, which is equipped with small wells for seeding cells, we can reduce the number of cells required to test a treatment. This is a decisive innovation to increase the number of drugs that can be tested”, adds Manzano.
A fast and fully automated system
The paper published in the journal npj Precision Oncology is the first to apply microfluidics to perform the functional assay of the DBP. Unlike other versions developed so far, such as the high-throughput DBP (Bhola et al., Science Signaling, 2020) with automated plates and dispensers to test hundreds of treatments, the new μDBP device is aimed at testing treatments in situ in a very fast, simple and automated way, without needing expensive machinery or specialised staff.
“The biggest advantage of the μDBP device is also the automation of the whole process, which would help to implement this functional methodology on a clinical scale. All these advantages would ease the adoption of DBP in hospitals as a routine trial”, the experts add.
“We have developed this new tool with the idea of making it available to oncologists. This automated system allows us to obtain personalised patient and treatment information”, says Javier Ramón-Azcón (IBEC).
Nanotechnologies: a revolution in biomedicine
Nanotechnology —especially the application of microfluidics to different processes— is driving several improvements in the design of these devices to reduce the amount of reagents, lower economic costs, automate processes or to increase the analytical capacity of certain methodologies, such as the DBP.
The team is currently working on the design of a new prototype with further technical improvements to facilitate the DBP analysis and to obtain more experimental evidence with primary samples to show its clinical utility in improving the treatment of different cancers, both paediatric and adult.
As the team details: “We will continue to work with our clinical collaborators to analyse patient samples and adapt this methodology to improve personalised treatment of multiple cancers for the benefit of all cancer patients”.
Reference article:
Manzano-Muñoz, A.; Yeste, J.; Ortega, M.A.; Martín, F.; López, A.; Rosell, J.; Castro, S.; Serrano, C.; Samitier, J.; Ramón-Azcón, J.; Montero, J. “Microfluidic-based dynamic BH3 profiling predicts anticancer treatment efficacy”. npj Precision Oncology, December 2022. Doi: 10.1038/s41698-022-00333-0





