Breast cancer is the most diagnosed cancer in women, with around 2.3 million new cases in the world every year, meaning that 1 out of 12 women will suffer from this disease during their lives. Despite its high incidence, breast cancer remains a challenge for clinicians and researchers, as due to the great complexity of tumour tissue and its “microenvironment”, it is very difficult to reproduce the actual conditions in the laboratory for study and treatment.
Now researchers at the Institute for Bioengineering of Catalonia (IBEC) have developed a new model of breast cancer that recreates the complexity of breast tumour composition. It is a ‘bioink’ consisting of pig breast tissues that have been deprived from cells, and which forms the basis for the growth of human cancer cells. The work, led by Elisabeth Engel, leader of the research group “Biomaterials for Regenerative Therapies” and Professor at the Polytechnic University of Catalonia (UPC), has just been published in the scientific journal ACS Appl. Mater. Interfaces.
3D tumour printing using the new bioink from pig breasts
A tumour is formed by cancer cells, which behave and divide abnormally and are immersed in the microenvironment surrounding them. The tumour microenvironment is formed by the extracellular matrix (ECM), a variety of epithelial cells, immune system cells, fat cells (adipocytes), soluble growth factors, and hormones, among others. The extracellular matrix is an important part of this microenvironment, as it is involved in tumour growth and drug efficacy. Furthermore, its complexity is one of the most restricting factors in the study of breast cancer.
The extracellular matrix is a highly complex type of support with very precise mechanical, biological and chemical characteristics, where cancer cells develop. By making a comparison with a nest of birds, the straw, leaves, branches, and other materials that form the nest would be the ECM that allows the development of eggs, in this example, cancer cells.
In order to recreate this complex ECM, IBEC researchers have used female pig breast tissue, taking advantage of the great similarity between the pig and human genomes and that it is a tissue that can be obtained easily and in large numbers. Through various procedures they have removed pig cells from breast tissue, leaving only ECM. This material, which they call bioink, is the basis for the next steps and the development of the tumour in the laboratory.
We have been able to develop, for the first time, a bioink from breast tissues devoid of their cells capable of mimicking the mechanical and biochemical characteristics of the extracellular matrix of human breast.”
Barbara Blanco-Fernández, first author of the study
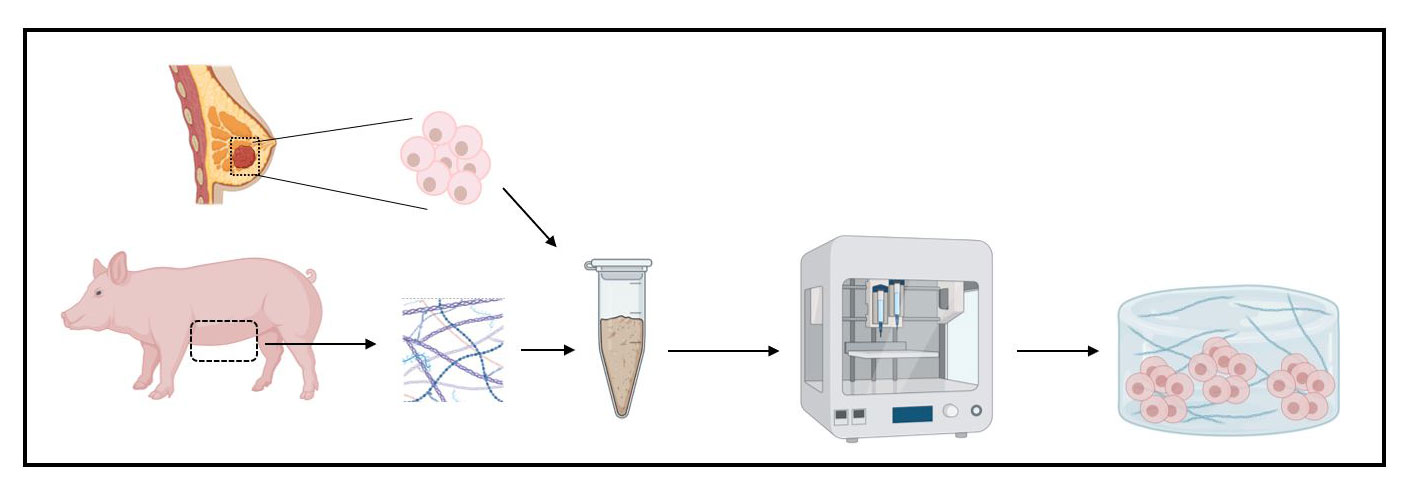
Once researchers have achieved this bioink, made up of the ECM of pork breast tissue, they have used it to generate a human breast tumour through 3D bioprinting. For this, to the bioink they added human cancer cells, factors such as type 1 collagen (present in large quantities in breast tumours) and other components that maintain the ideal structure and stiffness of the tumour. This mix is printed in 3D and after a few days of incubation under accurate and well-controlled conditions, a human breast tumour is obtained.
The new bioink developed by IBEC researchers highlights the importance of recreating the complexity of the extracellular matrix and at the same time the great potential of these materials for 3D bioprinting fabrication of tumour models for cancer study. In addition, this model opens the door to the development of more efficient therapies and personalized treatments, as tumours can be produced using patient cells.
Elisabeth Engel is member of the Centro de Investigación Biomédica en Red, Área de Bioingeniería, Biomateriales y Nanomedicina (CIBER- BBN) and the Red de Terapias Celulares (TerCel).
Reference article: Barbara Blanco-Fernandez, Sergi Rey-Vinolas, Gülsün Bağcı, Gerard Rubi-Sans, Jorge Otero, Daniel Navajas, Soledad Perez-Amodio, Elisabeth Engel. Bioprinting Decellularized Breast Tissue for the Development of Three-Dimensional Breast Cancer Models. ACS Applied Materials & Interfaces 2022 14 (26), 29467-29482.





