Cell division is a hallmark of life, and is essential for an organism’s growth, reproduction and repair. It is a highly intricate process driven by a precise cocktail of components. Unravelling the secrets of this phenomenon, and replicating it, could open up a host of potential biomedical applications. This is one of the ultimate goals of bottom-up synthetic biology – a field that aims to design and create synthetic cells that mimic nature. It will still be some time before we have fully functional synthetic cells, but a new study led by IBEC researchers has brought this goal one step closer. The paper, published in the scientific journal Advanced Materials, describes the integration of cell division machinery in synthetic cells, a breakthrough in the field.
The study was co-led by César Rodriguez-Emmenegger, group leader of IBEC’s Bioinspired Interactive Materials and Protocellular Systems group, and an ICREA Research Professor. Other IBEC researchers who contributed to the work include Marina I. Giannotti and Ricardo A. Zamora, both from the Nanoprobes and Nanoswitches group. The study was carried out in collaboration with researchers from the DWI–Leibniz Institute for Interactive Materials, the Max Planck Institute of Biochemistry, and the Centro de Investigación Biomédica en Red – Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN).
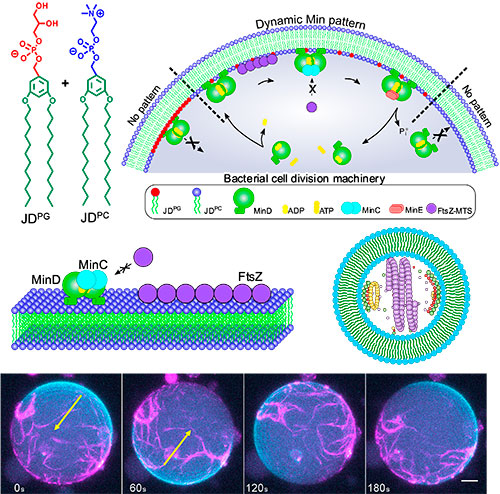
Replicating bacterial division
In bacteria, self-replication is carried out thanks to a highly complex protein system called the divisome. It creates a ring around the middle of the cell, which then constricts and cuts the cell in two. Researchers have tried to mimic this process in a variety of systems, but they have only succeeded when the system was built from lipids – the natural components that make up cell membranes. Now, for the first time, this study has managed to successfully insert the divisome into a completely synthetic material.
Over millions of years, evolution has crafted cell membranes that meet the requirements for life, so the researchers carefully studied these properties in order to replicate them in their synthetic cells. They assembled molecules called Janus dendrimers into a synthetic membrane and delicately tuned it until its biophysical properties were compatible with natural cellular processes. One of these properties was width, since the divisome proteins won’t attach if the membrane doesn’t have the right thickness. Another is charge, as the balance of attraction and repulsion between the proteins and the membrane is crucial. Finally, the membrane needs to be highly mobile, flexible and stable, so that it can withstand the forces exerted on it by the proteins.
Once they had calibrated the properties, they introduced the divisome protein complex to see if it would be compatible with their synthetic membrane. “What we observed was that, even though our molecules are completely different from lipids, we were able to extremely accurately reproduce the behaviour of the divisome, and this is of course a step closer to achieving self-replication in a synthetic cell”, says César Rodriguez-Emmenegger.
This study has demonstrated that synthetic entities can be designed to make use of the mechanisms provided by nature. Integrating cell division machinery is just the first step. Researchers are confident that this could become a powerful tool with many potential applications. “We were able to insert the machinery responsible for cell division by tuning our synthetic membrane. By the same token, in the future we should be able to incorporate any active cell machinery with the right adjustments”, points out Anna Wagner, a doctoral researcher in the Bioinspired Interactive Materials and Protocellular Systems group and first author of the study.
A new avenue to improve medicine
Synthetic cells could open up a host of possibilities in biomedicine, since they can be adjusted to perform functions that can’t be performed by normal cells. For example, a synthetic cell could be designed to mimic a macrophage – a type of white blood cell that engulfs pathogens – and fine tuned to specifically act on cancer cells, viruses or bacteria, especially those that are resistant to currently available drugs. Synthetic cells would have the advantage of not having the biological needs of living cells, and would not pose the same dangers as certain drugs.
“What excites me most about this work is not what we have achieved, but the prospect for the future. We have shown that we are able to copy the blueprint of nature – and there is no better engineer than nature”, concludes Rodriguez-Emmenegger.





