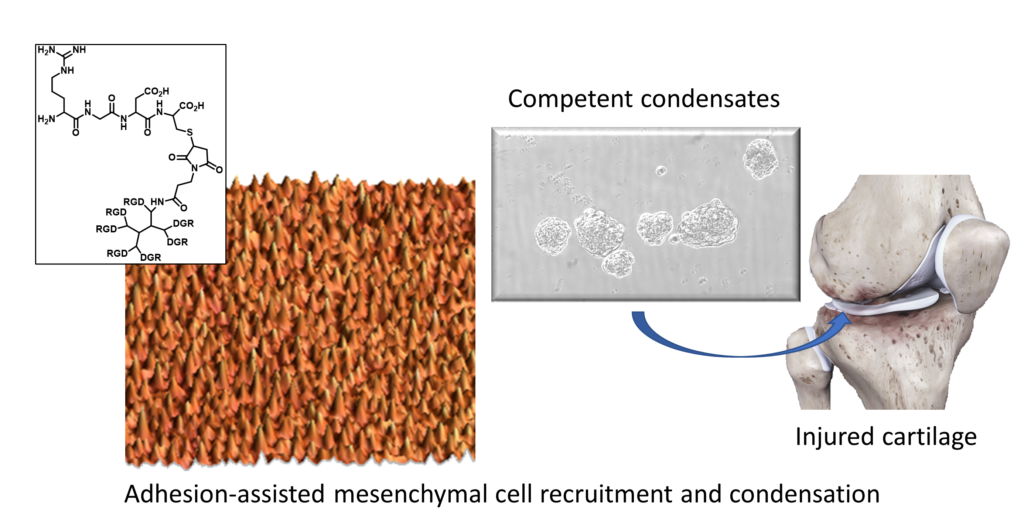
The World Health Organization estimates that more than 300 million people in the world suffer osteoarthritis each year. This disease entails a progressive tear and degradation of cartilage in the joints, leading to chronic pain and impaired mobility. One of the methods that currently exist to repair these injuries is to implant chondrocytes (cartilage cells) extracted from the patient. However, this technique is limited by the cell expansion step, required to generate large-sized tissues to fill the lesion, because very often chondrocytes dedifferentiate while in culture. Now, the IBEC Nanobioengineering research group, led by Professor Josep Samitier, publishes in the International Journal of Molecular Sciences a research work that deals with the cartilage generation in vitro using human mesenchymal stem cells (MSCs). These cells must pre-differentiate in vitro to successfully guide the differentiation into chondrocytes when inserted in the patient, a process that can be controlled by precisely tuning the cellular adhesion to the cell culture substrate.
In the group we develop tools to modulate cell-substrate interactions at the nanoscale to decipher their impact on tissue generation and development of diseases”.
Ignasi Casanellas,
first author of the work and PhD student at the laboratory of Professor Josep Samitier.
The nanopattern structure determines correct cartilage formation
MSCs are multipotent cells that differentiate, among others, into chondrocytes. Cartilage formation starts with mesenchymal cell condensation, in which MSCs migrate towards each other to form three-dimensional cell aggregates. In this work, mesenchymal condensation is improved by tailoring the level of adherence between MSCs and the underlying substrate at the nanoscale. This promotes directional cell migration, contributes to the mechanical stability of cell aggregates and improves cell-to-cell interactions, altogether leading to better cartilage formation in vitro. Researchers used MSCs derived from human adipose tissue, an easily accessible source for potential clinical applications.
The objective of this work is to be able to repair cartilage lesions using MSC easily extracted from the patient, which will be inserted in the lesion site after passing the pre-conditioning process in vitro.
Anna Lagunas,
senior researcher at the Nanobioengineering group from IBEC.
The group of Prof. Samitier has used nanopatterns, substrates for cell culture, of RGD (a tripeptide sequence that exists in several proteins from the extracellular matrix and is responsible for the cellular adhesion) to control and modify MSCs adhesion during the early steps of cartilage formation. By modifying the amount and spacing between the RGD motifs in the nanopatterns, they determine that there is more differentiation when using nanopatterns S90 (those presenting 90% of their surface with a distance between RGD motifs lower than 70 nm). These biocompatible nanopatterns, previously developed in collaboration with the Biomimetic Dendrimers and Photonic Laboratory from the Andalusian Centre for Nanomedicine and Biotechnology (Bionand), allow adjusting cell-substrate adhesion and can be generated on large surfaces, thus being compatible with tissue culture. Previous results from the group indicated that S90 nanopatterns also promote the differentiation process in vitro. The new findings show that high adhesion levels are needed to promote the condensation and viability of MSC aggregates, highlighting the importance of controlling cell–substrate adhesion in the tissue engineering strategies for cartilage repair.
The work was done in collaboration with different groups from the CIBER-BBN (Bioengineering, Biomaterials and Nanomedicine Networking Biomedical Research Centre): Tissue Regeneration and Bioengineering Laboratory, University of Málaga (LABRET-UMA).
Reference article: Ignasi Casanellas, Anna Lagunas, Yolanda Vida, Ezequiel Pérez-Inestrosa, José A. Andrades, José Becerra, Josep Samitier. The Janus Role of Adhesion in Chondrogenesis. Int. J. Mol. Sci. 2020.





