A new triple-acting antibiotic agent has managed to break through the biofilm extracellular matrix – a protective structure built by bacteria – and eliminate more than 50% of the pathogens in one shot. The study, published in the scientific journal npj Biofilms and Microbiomes, was led by Eduard Torrents, group leader at the Institute for Bioengineering of Catalonia (IBEC).

Persistent lung infections, chronic wounds, and healthcare-associated infections are typically much more difficult to treat than other types of bacterial infections. This is because they are often caused by biofilms – colonies of microbes, mainly bacteria, that grow together in a self-produced matrix that protects and isolate them from the external environment.
This extracellular matrix exacerbates antibiotic resistance —“one of the biggest threats to global health”, according to the World Health Organization— since it makes it up to 1000 times harder to kill the bacteria inside the biofilm. Biofilm infections are therefore the most important nonspecific mechanism of antimicrobial resistance.
“By combining an antibiotic with a pair of biofilm-piercing agents, we built a drug that’s far more potent than the antibiotic on its own – eliminating even the bacteria living inside the biofilm,” explains Eduard Torrents, chief researcher of this work.
Attacking these microbes with antibiotics alone is not enough. There is a need for tools that break down the extracellular matrix to access and kill the bacteria inside. A groundbreaking study published in the scientific journal npj Biofilms and Microbiomes, and led by the Institute for Bioengineering of Catalonia (IBEC), has achieved this.
Torrents leads the Bacterial infections: antimicrobial therapies group at IBEC and is an associate professor at the University of Barcelona. Núria Blanco-Cabra, first-author of the scientific paper, is a postdoctoral researcher in Torrents’ group at UB/IBEC. The research was carried out in collaboration with scientists at CIDETEC (Basque Country).
Triple-acting drug combination
In this study, the bacteria investigated was Pseudomonas aeruginosa. This pathogen commonly grows in biofilms in the lungs of patients with cystic fibrosis or chronic obstructive pulmonary disease (COPD), causing persistent infections. ”We grew biofilm cultures in vitro, using a technique that closely resembles the way they exist and grow in nature”, added Torrents. In clinical practice these infections are usually treated with an antibiotic called tobramycin. However, its effectiveness is limited by its inability to penetrate the biofilm. This is because tobramycin, which is positively charged, is neutralized by the negatively charged extracellular matrix.
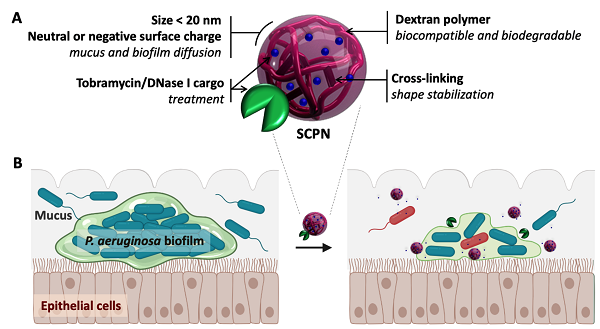
With this in mind, the researchers loaded the antibiotic into negatively charged nanoparticle carriers. This had the effect of neutralizing the positive charge before the drug reached the biofilm, allowing it to break up the extracellular matrix and kill the bacteria inside. Crucially, these carriers – made from dextran-based single-chain nanoparticles – were able to carry up to 40% of the weight of the antibiotic. “Many previously-studied nanocarriers have only been able to sustain a small load of the target compound, which has prevented their clinical use. We managed to overcome this hurdle”, says Torrents.
The antibiotic-loaded nanocarriers were also coated in an enzyme called DNase I. One of the “glues” that holds bacteria biofilms together is structural DNA found throughout the extracellular matrix. DNase I is able to break down this glue, causing the matrix to loosen, and allowing the antibiotic to even further penetrate the biofilm. By observing a series of time-lapse microscopy images, the researchers were able to verify that their agent had not only dissolved the structural DNA in the extracellular matrix, but also that it was acting on and killing the bacteria inside. With just one application, they reduced the bacterial biomass by more than half.
New hope for “untreatable” infections
Having achieved such a significant clearance of the biofilm after just one dose of our agent, we predict that a full antibiotic course could considerably reduce the burden of these extremely difficult-to-treat infections.
Eduard Torrents, IBEC
In clinical use, this agent would be administered in multiple doses, as is standard practice with antibiotics.
The next step is to work towards the clinical validation of this system. Its commercialization would represent a critical step forward in the treatment of biofilm infections – for which the global economic toll currently stands at US$4,000 billion per year.
Reference article:
Núria Blanco-Cabra, Julie Movellan, Marco Marradi, Raquel Gracia, Cristian Salvador, Damien Dupin, Iraida Loinaz & Eduard Torrents. Neutralization of ionic interactions by dextran-based single-chain nanoparticles improves tobramycin diffusion into a mature biofilm. npj Biofilms Microbiomes 8, 52 (2022). https://doi.org/10.1038/s41522-022-00317-9





