ABOUT
The main goal of the Nanoscale Bioelectrical Characterization group is to develop a multiscale and multimodal (electrical, mechanical) approach to Bioelectricity, covering from the nano- to the microscale. To this end the group combines methods and techniques from Scanning Probe Microscopy, Artificial Intelligence and Organic Bioelectronics. The main objective is to contribute to develop new label-free characterization tools for Life Sciences, new nanomedical diagnosis approaches and new electronic biosensors.
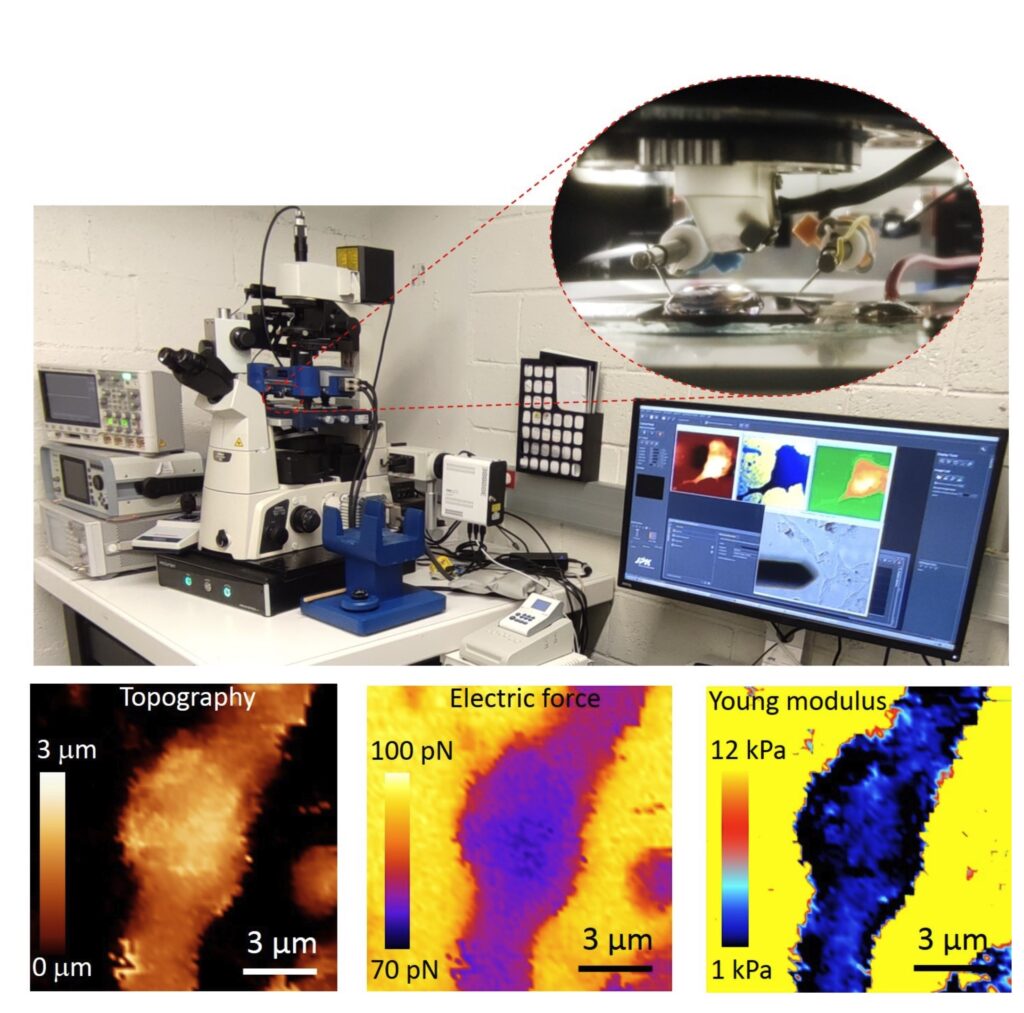
Autonomous multimodal scanning probe microscopes for Life Sciences
At present the group focuses in the development of an Autonomous Multimodal Functional Scanning Probe Microscope assisted by Artificial Intelligence for Life Sciences and Medical applications. The objective is to map the structural, electrical and mechanical properties at the nanoscale of cells, bacteria, drug nanocarriers and organic Bioelectronic devices with minimal intervention of the operator and at high throughput.
The objective is to obtain in an autonomous way fast functional electric and mechanical nanoscale maps of Life Science samples and Organic Electronics devices in physiological conditions with minimal intervention of the operator and at high throughput.
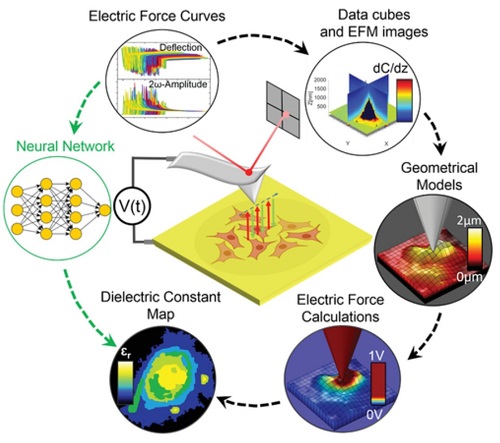
Initial results obtained by the group include the upgrade of the Scanning Dielectric Microscope to enable its operation in physiological buffers for living cell imaging, the development of a supervised machine learning algorithm to process Scanning Dielectric Microscopy data and provide almost instantaneously local dielectric constant maps of both eukaryotic and prokaryotic cells, and the implementation of a workflow for Scanning Dielectric Microscopy for high throughput and automatic nanoscale multimodal (electrical and mechanical) characterization.
High throughput multimodal characterization of drug nanocarriers
The development of novel drug nanocarriers require an exhaustive multiparametric characterization, which includes its morphology and structure, net charge, particle size distribution or phase transition temperature. These characteristics are obtained usually from different techniques. We target to obtain simultaneously and at high throughput multiparametric information on drug nanocarriers by using a single instrument, namely, the autonomous multimodal in liquid Scanning Dielectric Microscope. We aim at obtaining information on the size, sphericity, membrane wall thickness, lamellarity, Young’s modulus, stiffness, surface charge and membrane specific capacitance of drug nanocarriers, such as liposomes, polymeric nanoparticles or lipid nanoparticles.
Interrelation of mechanical and electrical processes in living neurons
Mechanical and electrical processes in cells and tissues can sometimes appear interrelated, as for instance, in the action potential propagation in neurons, which provokes the electrical polarization of the cell membrane and, at the same time, a change in neuron’s membrane tension. Similarly, the restructuring of the cytoskeleton of neurons, as occurring in the Alzheimer disease, can induce a change in cellular stiffness and, consequently, an improper neuron firing. We aim at investigating this interrelation by means of the multimodal in liquid Scanning Dielectric Microscope applied to living neurons.
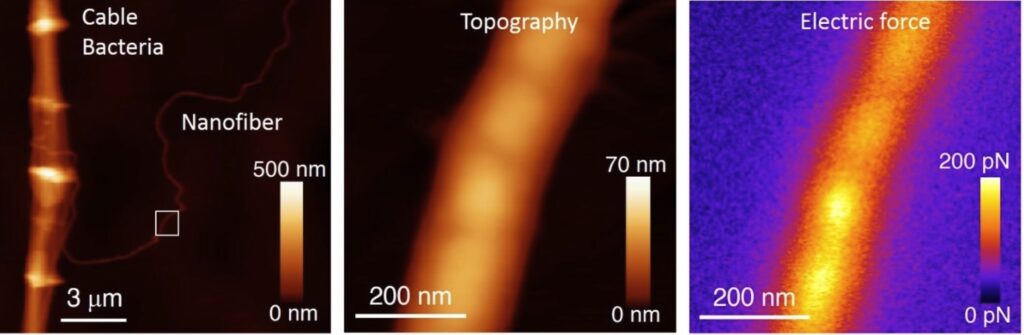
Unravelling the electrical conduction properties of cable bacteria
Long-range electron conduction in cable bacteria filaments presents unusual characteristics in the biological world, exceeding by more than 6 orders of magnitude the conductivity of the best conducting protein nanowires. Electric conduction takes place through Niquel rich protein nanofibers located in the bacteria periplasm, but still many aspects of the electronic conduction in cable bacteria remain unknown. We aim at providing new insights on the conducting properties of cable bacteria by using the unique capabilities and versatility of the Scanning Dielectric Microscope.
Novel nanoscale physical phenotyping of cancer cells
The whole process of cancer aggression, from local growth to extravasation into blood vessels, migration, seeding into different organs and formation of metastases involves physical changes (mechanical and electrical) and their interplay with protein expression and genetic transformations. We aim at developing a high throughput nanoscale multimodal physical phenotyping method for cancer cells based on the Scanning Dielectric Microscope. Our ling term objective is to provide additional diagnostics tools to medical doctors for evaluating cancer progression and aggression.
Structure-function relationships for materials in Organic Bioelectronics
Organic semiconductor materials have emerged as key materials in the development of platforms (e.g. electrolyte gated transistors) for transducing and amplifying biological and biochemical signals. This fact makes them an integral part of diverse biosensing and bioelectronic devices able to sense even single molecules or to record bioelectric potentials from excitable cells. The fundamental understanding of the nanoscale electronic and ionic transport governing the operation of these materials and devices remains, however, poorly understood. We aim at providing new insights into the structure-function relationship of organic materials used in Bioelectronics with the unique capabilities of the multimodal in operando in-liquid Scanning Dielectric Microscope.
STAFF
Staff members:
Former members:
Harishankar Balakrishnan | PhD Student
Now: Post-doc, University of Munich (Germany)
Ignacio Casuso | PhD Student
Now: Staff Scientist, INSERM (France)
Maria Chiara Biagi | PhD Student
Now: In-vivo Image Analysis Scientist, AstraZenca (Spain)
Marti Checa | PhD Student
Now: R&D Staff scientist, Oak Ridge National Laboratory (USA)
Martin Edwards | Postdoc
Now: Assistant Professor, University of Arkansas (USA)
Daniel Esteban Ferrer | PhD Student
Now: CEO, ViR S.L. (Spain)
Laura Fumagalli | Senior Researcher
Now: Reader, University of Manchester (UK)
Georg Gramse | PhD Student
Now: Group Leader, Johannes Kepler University of Linz (Austria)
Larisa Huetter | PhD Student
Now: IT consultant, Rewion (Germany)
Adrica Kyndiah | Postdoc
Now: Senior Scientist, Instituto Italiano di Tecnologia (Italy)
Helena Lozano | PhD Student
Now: Project Manager, CSIC (Spain)
Martina di Muzzio | PhD Student
Now: Engineer PMQ, Roche (Spain)
Jordi Otero | Postdoc
Now: Lecturer, Universitat de Barcelona (Spain)
Shubham Tanwar | PhD Student
Now: Post-doc, Italian Institute of Technology (Italy)
Romen Trujillo | PhD Student
Now: Associate Professor, Universitat de Barcelona (Spain)
Marc Van der Hofstadt | PhD Student
Now: Post-doc, CNRS (France)
PROJECTS
| INTERNATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| PRINGLE · Protein Based Next Generation Electronics (2022-2026) | European Commission, PathFinder Open | Gabriel Gomila |
| SPM4.0 · Autonomous Scanning Probe Microscopy for Life Sciences and Medicine powered by Artificial Intelligence | European Commission , MSCA-DN 2023 | Gabriel Gomila |
| NATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| ICREA Academia Award (2023-2027) | Catalan Institution for Research and Advanced Studies (ICREA) / Generalitat de Catalunya | Gabriel Gomila |
| Microscopio de fuerzas de barrido multiparamétrico autónomo y de alto rendimiento para aplicaciones en ciencias de la vida y medicina (BIOMEDSPM4.0) | MICIU/AEI and FEDER, UE | Gabriel Gomila |
| SGR-Grups de recerca consolidats (SGR-Cat 2021)_GRC | AGAUR / SGR | Gabriel Gomila |
| FINISHED PROJECTS | FINANCER | PI |
|---|---|---|
| SGR Grups de recerca consolidats (2017-2020) | AGAUR / SGR | Gabriel Gomila |
| SPM2.0 · Scanning probe microscopies for nanoscale fast, tomographic and composition imaging (2017-2020) | Marie Curie Skłodowska European Training Network (MSCA-ITN-ETN) | Gabriel Gomila (Project Coordinator) |
| NANOMICROWAVE · Microwave Nanotechnology for Semiconductor and Life Sciences (2013-2016) | MARIE CURIE – ITN | Gabriel Gomila |
| V-SMMART Nano · Volumetric Scanning Microwave Microscopy Analytical and Research Tool for Nanotechnology (2012-2016) | NMP – SME | Gabriel Gomila |
| AFM4NanoMed&Bio · European network on applications of Atomic Force Microscopy to Nanomedicine and Life Sciences | EU COST Action TD1002 | Gabriel Gomila (Management Committee Substitute Member) |
| BIOWIRESENSE · Plataforma universal para la detección de biomarcadores basada en nanocables bacterianos conductores (2017-2019) | MINECO, Explora Ciencia | Gabriel Gomila |
| NANOELECTOMOGRAPHY· Electrical nanotomography based on scanning probe microscopy for nanomaterials and biological samples (2014-2016) | MINECO (TEC2013-48344-C2-1-P) | Gabriel Gomila |
| NANOELECTROPHYS · Scanning Electric Force Microscope for Electrophysological Recordings at the Nanoscale (2016-2019) | MINECO (TEC2016-79156-P) | Gabriel Gomila |
| ICREA Academia Award (2015-2019) | Catalan Institution for Research and Advanced Studies (ICREA) / Generalitat de Catalunya | Gabriel Gomila |
| BORGES · Biosensing with ORGanic ElectronicS (2019-2022) | Marie Curie Skłodowska European Training Network (MSCA-ITN-ETN) | Gabriel Gomila |
| BIGDATASPM · Métodos de datos masivos aplicados a la Microscopía de Sonda de Barrido para estudios eléctricos funcionales en ciencias de la vida (2020-2023) | MINECO, Generación Conocimiento: Proyectos I+D | Gabriel Gomila |
| Correlative Electrical and Mechanical Scanning Probe Microscopy for Life Science Application | Beatriu de Pinós 2019/ AGAUR | Aurora Dols |
PUBLICATIONS
EQUIPMENT
- Cypher Atomic Force Microscope (Asylum Research)
- Nanowizard 4 Bio-Atomic Force Microscope (JPK)
- Cervantes Atomic Force Microscope (Nanotec Electronica)
- Easy Scan 2 Atomic Force Microscope (Nanosurf)
- AxioImager A1m Reflection Optical Microscope (Zeiss) equipped with a AxioCam ERc5s (Zeiss)
- CompactStat portable electrochemical interface and impedance analyzer (Ivium Technologies)
- Palmsens 4, 8 channel Potentiostat (Palmens)
- 2 eLockIn204 4-phase Lock-In amplifiers (Anfatec)
- Keithley 6430 sub-femtoAmp remote sourcemeter
- Keysight B2912A precision Source/Measure Unit, 2 channels
- Keysight N9310A RF Signal Generator 9 kHz to 3.0 GHz
- Computation Workstation Intel Xeon, NVIDIA RTXA5000
COLLABORATIONS
- Dr. Filip Meysman
University of Antwerp, Belgium - Dra. Adrica Kyndiah
Italian Institute of Technology, Italy - Dr. Martí Checa
Oak Ridge National Laboratory, USA - Dr. Jordi Borrell
University of Barcelona, Spain - Dra. Marta Mas-Torrents
Institut de Ciències de Materials de Barcelona, Spain - Dr. Eduard Torrents
Institut de Bioenginyeria de Catalunya, Spain - Dr. Jose Antonio del Rio
Institut de Bioenginyeria de Catalunya, Spain
NEWS
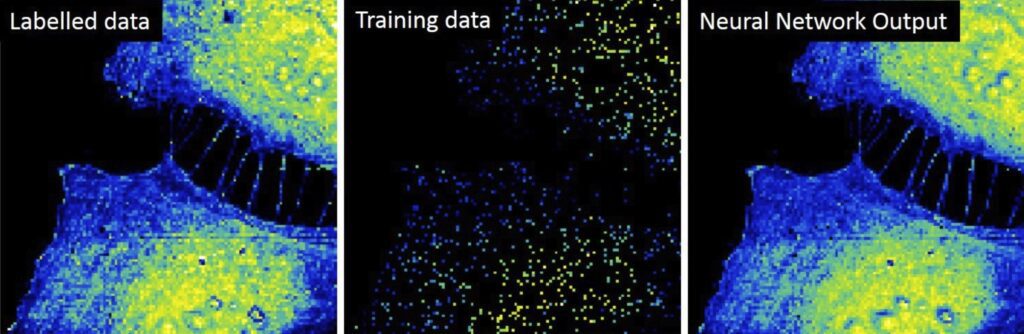
Machine learning reduces microscope data processing time from months to just seconds
With a new method that combines high-powered scanning force microscopes and machine learning, IBEC researchers have drastically reduced the processing time required to achieve nanoscale biochemical compositions map from electric images of eukaryotic cells in just seconds. Using earlier computation methods, processing one image could take even months.

Two IBEC researchers receive a Beatriu de Pinós fellowship
Aurora Dols and Zaida Álvarez, researchers at the Institute for Bioengineering of Catalonia (IBEC), receive the prestigious fellowships Beatriu de Pinós, awarded by the Catalan Government for the incorporation of highly qualified postdoctoral researchers into the Catalan research system.
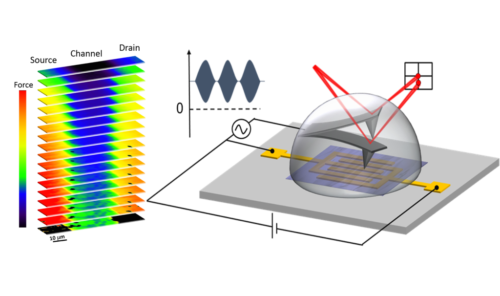
Electric forces to characterize future biocompatible organic electronic devices
A joint collaboration between the Institute for Bioengineering of Catalonia (IBEC), the Institute of Materials Science of Barcelona (ICMAB) and The University of Manchester has succeeded in mapping the electrical properties of organic biosensor/electrolyte interfaces at the nanoscale by measuring local electric forces. Electronic biosensors based on organic materials could make soon a reality the dream of low-cost, disposable, flexible and biocompatible electronic devices for the interaction with biological systems .
A research team develop biotransistors able to hear small beats of live
Researchers at IBEC and ICMAB develop a flexible, cheap and biocompatible transistor platform able to record an electrocardiogram of cells and micro-tissues during long periods of time. The platform, based on organic transistor technology (EGOFET), can also measure the effect of drugs on beating cells, as cardiomyocytes, opening the door to several applications such as implantable devices for health.

Water can be dead, electrically speaking
Research led by the University of Manchester’s National Graphene Institute, with the collaboration with IBEC, reveals that water that’s only a few molecules thick – like the water that covers every surface around us – behaves very differently to normal, ‘bulk’ water. Water is one of the most fascinating substances on Earth. At the heart of its many unusual properties is its high polarizability – that is, its strong response to an applied electric field.

Training the next generation of advanced microscopy experts
An IBEC group has been awarded EU funding to coordinate a project that aims to train a new generation of researchers in the science and technology of Scanning Probe Microscopes. Thanks to the Marie Curie ITN funding, the ten consortium members of the SPM2.0 European Training Network – located in Spain, France, Austria, the UK and Italy – will be able to provide researchers with state-of-the-art multidisciplinary scientific training in the field of Scanning Probe microscopies, covering basic science to industrial applications, which should enable them to generate new scientific knowledge.
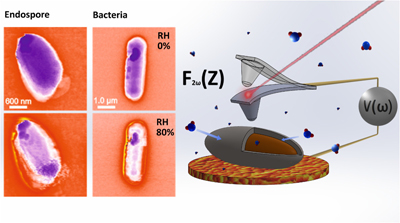
Using EFM to probe the secrets of bacterial endospore survival strategies
An IBEC group has demonstrated, for the first time, that the hydration properties of a single bacterial endospore in varying environmental relative humidity can be determined with high accuracy and reproducibility, and in a non-destructive way, shedding new light on endospore survival strategies. Endospores are recognized as the hardiest form of life on Earth, and are produced by certain bacterial cells in response to a lack of nutrients.


 ibecbarcelona.eu
ibecbarcelona.eu