Coinciding with World Diabetes Day, researchers from the Institute for Bioengineering of Catalonia (IBEC) reveal a study that combines muscle and pancreatic cells on a single chip and demonstrates that insulin production by the pancreas during exercise is induced by the contraction of muscle cells.
The new chip is a powerful tool for diabetes drug development and testing, and it allows personalised modelling of the disease by using patient’s own cells. With this model, researchers and clinicians will have the possibility to study diabetes following a new approach, by combining different cell types, and study in detail why insulin production by the pancreas sometimes fails.
According to the World Health Organisation, 422 million people worldwide suffer from diabetes mellitus, and 1.5 million deaths each year are directly associated with the disease. Diabetes is a complex metabolic disease characterised by a high accumulation of glucose in the blood resulting from a failure in the production or activity of insulin. Insulin, in turn, is a hormone produced, stored and released by the pancreas, which enables glucose to enter the different cells of the body to provide them with the energy they need to function properly.
Muscle is one of the main targets of insulin and is crucial in the overall maintenance of glucose levels throughout the body. Current physiological models for in vivo study in humans or animals allow researchers to study the effect of physical exercise on insulin secretion, but not to determine whether that effect is due to muscle contraction or to some other organ mediating this signalling. On the other hand, in vitro studies with traditional 2D cultures do not allow real-time analysis of the interaction between muscle cells and pancreatic cells, as they do not recreate the 3D structure of tissues and organs, which are highly relevant to their physiology.
The development of new drugs to prevent and treat diabetes must be based on tools that take into account the communication between the pancreas and other organs in order to recreate the details of the disease. In a recent study published in the Advanced Materials Technology journal, IBEC researchers led by Juan M. Fernández-Costa and Javier Ramon-Azcon from the “Biosensors for Bioengineering” group, have developed a “multi-organ-on-chip” that allows, in a single device, to study the communication between the insulin-producing cells of the pancreas and the muscle cells. By using this innovative “gym on a chip”, researchers found that muscle cells contraction caused by electric stimulation directly induces the production of insulin by pancreatic cells.
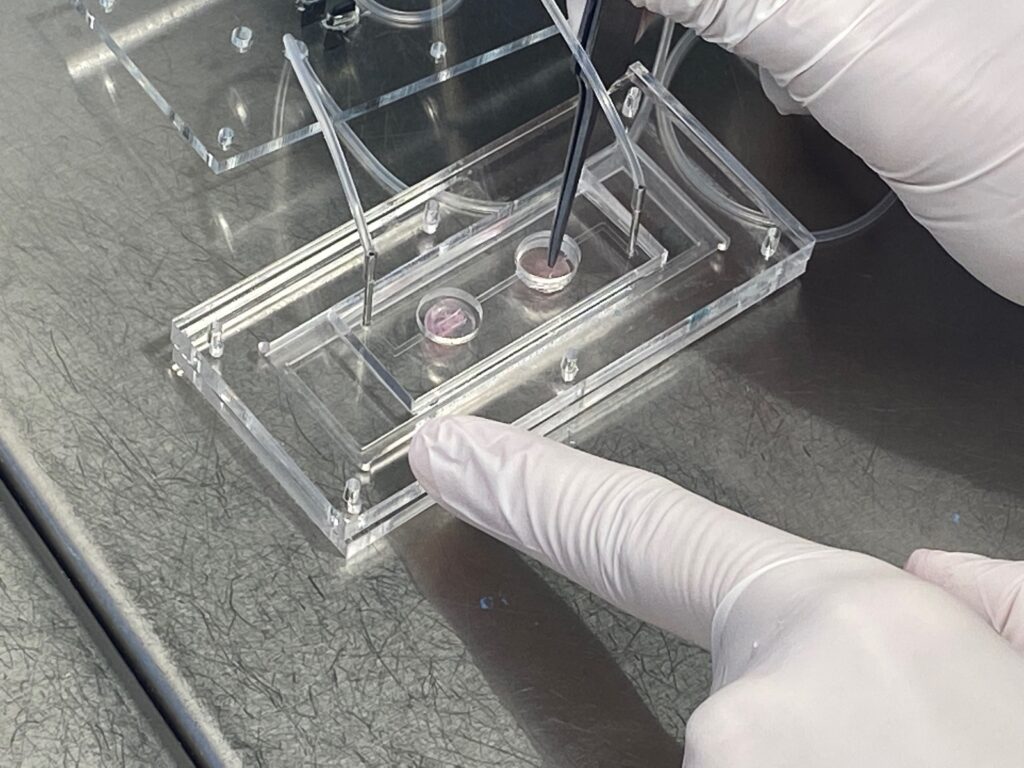
The “gym on a chip”
Inside this novel bioengineering device, IBEC researchers have placed in each one of the two compartments different cell types: skeletal muscle cells, the muscles that are attached to bones and provide us with mobility, and pancreatic cells, concretely pancreatic islets composed of beta cells, which produce insulin. As these cells grow and develop, they form 3D structures that simulate a “mini-organ” inside the chip.
The “multi-organ-on-chip” device has a system of tiny channels that allow both compartments to communicate in a controlled way and monitor, on demand and in different situations, the exchange of signals and compounds between the different cell types.
The muscle cells of the “multi-organ-on-chip” are connected to an electrical stimulator that simulates physical exercise and induces their contraction. In addition, the device is also integrated with a complex biosensor platform capable of monitoring insulin levels produced by pancreatic cells, and interleukin-6 (IL-6) levels without the need of external labelling. IL-6 is a regulatory protein that is produced by the muscle and signals the pancreas to increase insulin production. In other words, during exercise, IL-6 moves from the muscle to the pancreas to stimulate insulin production.
“With our “gym on a chip” we demonstrate that insulin secretion by pancreatic islets during exercise is dynamically mediated by the contractile activity of muscle cells and not by other intermediates”. Juan M. Fernández-Costa, first author of the study.
The future of “multi-organ-on-chip”
The new in vitro multi-organ platform developed by IBEC is coupled to a detection platform that has a number of advantages over current detection systems, as it has an affordable manufacturing cost and does not need the use of labelled antibodies. In addition, it can be easily connected to other organ-on-chip devices and enables highly sensitive real-time monitoring of the metabolism of the different organs involved.
The platform developed in this study has enabled in situ, real-time monitoring of pancreas-muscle communication in diabetes, and the study of insulin secretion dynamics by pancreatic islets in response to muscle exercise induced by electrical pulse stimulation. It represents a major step forward in the modelling of diabetes, the study of insulin resistance and the investigation of drug candidates for therapy. Moreover, this new tool will contribute to reducing the use of animals for experimentation, which is time-consuming, costly, ethically limiting, and make difficult to extrapolate results to humans.
“Organ-on-chip devices accurately represent organ physiology in vivo and allow the analysis of drug kinetics by studying functionality and communication between tissues”. Javier Ramon-Azcon, Biosensors for Bioengineering group leader
In the future, the “muti-organ-on-chip” could also be used to study communication between other organs in the context of various metabolic diseases, providing a promising platform for new drug screening and personalised medicine.
Reference article: Training-on-a-Chip: A Multi-Organ Device to Study the Effect of Muscle Exercise on Insulin Secretion in Vitro. Juan M. Fernández-Costa, María A. Ortega, Júlia Rodríguez-Comas, Gerardo Lopez-Muñoz, Jose Yeste, Lluís Mangas-Florencio, Miriam Fernández-González, Eduard Martin-Lasierra, Ainoa Tejedera-Villafranca, and Javier Ramon-Azcon. Adv. Mater. Technol. 2022, 2200873.
With the support of Joan Oró grant to hire research staff in training (FI 2025), 2022 FI_B 00386, funded by Generalitat de Catalunya and by the European Social Fund Plus.






