Researches from the Institute for Bioengineering of Catalonia (IBEC) and the University of Barcelona (UB) have achieved the creation of the first highly mature neurones from human induced pluripotent stem cells (iPSCs) using a synthetic material, opening up new opportunities for the medical research and potential therapies for neurogenerative diseases and traumatic lesions. The study has just been published in the journal Cell Stem Cell.
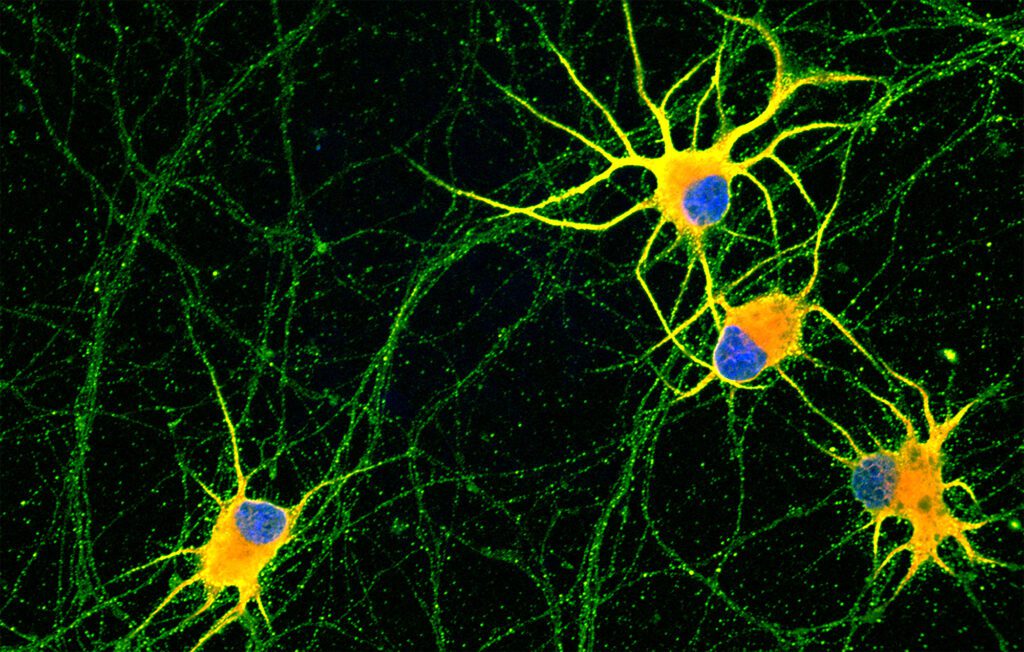
Until now, it has been possible the generation of neurons from induced pluripotent strem cells, but these neurones presented an insufficient maturation degree, similar to neurons in early stages of development. This limited its capacity to be used in researching neurogenerative diseases, since it is the adult neurons that degenerate. The inefficient maturation of neurons differentiated from iPSCs was partly due to the lack of signals found in the neurons’ environment, the extracellular matrix.
“This is the first time human iPSC-derived neurons have been matured with a synthetic matrix. This platform will allow laboratories to have mature human neurons to study multiple neurological diseases and develop new therapies”, comments Zaida Álvarez, Ramón y Cajal researcher at the Institute for Bioengineering of Catalonia (IBEC) and co-first author of the study.
The extracellular matrix is essential for the development of cells in the lab, since it provides structural support, regulates the signalling and cellular differentiation, keeps the integrity and proportionate an adequate environment for the cellular growth.
To recreate the extracellular matrix and achieve a functional maturation similar to adult neurons from the nervous system in physiologic conditions, the researchers used “dancing molecules”, a revolutionary technique presented this year by Dr. Zaida Álvarez from IBEC and the professor Samuel I. Stupp from the University of Northwestern .
The first step was to differentiate the human iPSCs into motor and cortical neurons to later place them on nanofibers composed of “dancing molecules”, where the researchers observed that the signaling and branching capacity of the neurons had improved, allowing them to generate better cells. synaptic contacts with each other.

The researchers believe that by advancing the age of neurons in cell cultures, experiments may be improved to better understand late-onset diseases. “Having mature neurons in the laboratory is essential to advance in the understanding of neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease or amyotrophic lateral sclerosis (ALS), and in the development of effective and safe therapies”, comments Alberto Ortega, Ramón y Cajal researcher at the Faculty of Medicine and Health Sciences of the University of Barcelona (UB), member of the Institute of Neurosciences of the UB (UBneuro) and co-first author of the study.
Synchronized “dance” skills
To grow the mature neurons, researchers used nanofibers composed of “dancing molecules”, a material that Zaida Álvarez developed at Stupp’s laboratory as a potential treatment for acute spinal cord injuries. In previous research published in the journal Science, Zaida Álvarez discovered how to change the movement of molecules so they can find and connect more efficiently to the cellular receptors that are constantly moving.
In the new study, Zaida Álvarez and Alberto Ortega found that nanofibers with higher molecular motion led to improvements in cultures of human neurons. In other words, neurons grown on these more dynamic synthetic materials showed greater maturity, less aggregation, and more intense signalling.
“We believe that this works because receptors are moving very fast on the cell membrane and the signalling molecules of our scafolds are also moving very fast”, explained Stupp, director of the Simpson Querrey Bionanotechnology Institute (SQI) and distinguished Severo Ochoa professor at IBEC.
As part of the research, they took skin cells from an ALS patient and turned them into patient-specific motor neurons, the cell type affected in this neurodegenerative disease. These neurons were cultured for two months on the synthetic materials to develop characteristics of the ALS disease. “Not only has this provided a new window to study ALS, but this system can also be used to study and test potential therapies in other neurological diseases,” says Evangelos Kiskinis, professor of neurology and neuroscience at the Feinberg School of Medicine at the Northwestern University and Robertson Investigator at the New York Stem Cell Foundation.
Hopes for future treatments for spinal cord lesions and neurodegenerative diseases
Later, these highly functional neurons, thanks to the synthetic material, could be transplanted into patients with loss of neurons, due to injury or disease, which could restore lost cognition or sensations. And, because the initial cells could come from the same patient, the derived and transplanted neurons would not generate rejection.
In this study, it have also participated Kohei Sato, researcher from the Life Science School and Technology from the Tokyo Institute of Technology, and Elisabeth Engel, principal researcher of the Biomaterials for regenerative therapies’ group from the Institute of Bioengineering of Catalonia (IBEC).
The study, “Artificial extracellular matrix scaffolds of mobile molecules enhance maturation of human stem cell-derived neurons,” was supported by The Spanish Ministry of Science, Mike Lane fellowship from Castellers de Vila de Gracia 2021, AFM-Telethon Trampoline, Beatriu de Pinos 2019, Ramon y Cajal fellowship, the Center for Regenerative Nanomedicine at the Simpson Querrey Institute for BioNanotechnology, the National Institutes of Health (NIH) National Institute on Neurological Disorders and Stroke, NIH National Institute on Aging, NIH National Institute of Biomedical Imaging and Bioengineers, NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Les Turner ALS Foundation, the New York Stem Cell Foundation, U.S. Department of Energy and the Paralyzed Veterans of America Research Foundation.
Reference article:
Zaida Álvarez, J. Alberto Ortega, Kohei Sato, Ivan R. Sasselli, Alexandra N. Kolberg-Edelbrock, Ruomeng Qiu, Kelly A. Marshall, Thao Phuong Nguyen, Cara S. Smith, Katharina A. Quinlan, Vasileios Papakis, Zois Syrgiannis, Nicholas A. Sather, Chiara Musumeci, Elisabeth Engel, Samuel I. Stupp and Evangelos Kiskinis. Artificial extracellular matrix scaffolds of mobile molecules enhance maturation of human stem cell-derived neurons. Cell Stem Cell (2023), DOI: https://doi.org/10.1016/j.stem.2022.12.010





