 Understanding the biological characteristics of biofilms, the cause of most known bacterial infections, is the first step to fight against this silent pandemic and to find effective treatments.
Understanding the biological characteristics of biofilms, the cause of most known bacterial infections, is the first step to fight against this silent pandemic and to find effective treatments.
Bacterial infections are a public health problem affecting millions of people worldwide, and about 80% of them are associated with biofilms, communities formed by different microorganisms’ species which co-colonize human tissues and medical devices. Due to several biological characteristics, biofilms are much more recalcitrant than single bacterial infections and need longer periods and higher antibiotic doses to be treated. This situation contributes increasing the overall antibiotic resistance we are observing nowadays in the clinic.
In this context, Pseudomonas aeruginosa is a bacterium that can coexist and interact with a broad range of microorganisms in biofilms causing numerous infections with a high clinical impact and often leading to chronic infections. Now, Eduard Torrent, leader of the “Bacterial infections: antimicrobial therapies” group at IBEC and professor at the Genetics, Microbiology and Statistics Department from the Faculty of Biology at the University of Barcelona (UB), and Maria del Mar Cendra, a postdoctoral researcher in his lab, publish in the Biotechnology Advances Journal, a broad review outlining key aspects of P. aeruginosa biofilms, including the main microorganisms species that may co-exist and the clinical burden they represent.
P. aeruginosa biofilms
The high ability of P. aeruginosa to grow in biofilms with fungi, viruses and other bacteria (it is almost never found infecting alone) and to colonize medical devices and human tissues is a growing global public health concern. Those biofilms have increased antibiotic tolerance and resistance to host defense responses. In this review, researchers decipher the implication of P. aeruginosa in biofilms and pave the way to find solutions in a near future.
Only with the continuous development and improvement of efficient antibiofilm strategies we can tackle the recurrence and chronicity caused by P. aeruginosa biofilm infections.
Eduard Torrents, leader of the “Bacterial infections: antimicrobial therapies” group at IBEC and professor at UB.
P. aeruginosa interacts with many respiratory viruses in chronic infections, including SARS-CoV-2, the causal agent of the COVID-19 pandemic. Studies from other researchers have shown that 7% of hospitalized patients infected with SARS-CoV-2 suffered from bacterial coinfection, of which P. aeruginosa appeared in 12% of the cases. Moreover, P. aeruginosa can colonize medical devices such as endo-tracheal tubes, used in COVID-19 patients that need assisted breathing. Biofilm formation has been found in 95% of patients intubated with endo-tracheal tubes for more than 24h. These kinds of biofilms, where P. aeruginosa is the primary causative agent, are perdurable and able to remain despite antibiotic treatment, increasing the risk of respiratory infections.
P.aeruginosa, together with Staphylococcus aureus and Streptococcus, other bacteria, also plays a leading role in biofilms’ infections associated with cystic fibrosis (40-60% of total), a genetic disorder that affects mostly the lungs, but also the pancreas, liver, kidneys, and intestine. It is also a relevant pathogen in chronic wounds infections and skin injuries, and in keratitis, a common corneal infection related with bacterial biofilm formation over contact lenses.
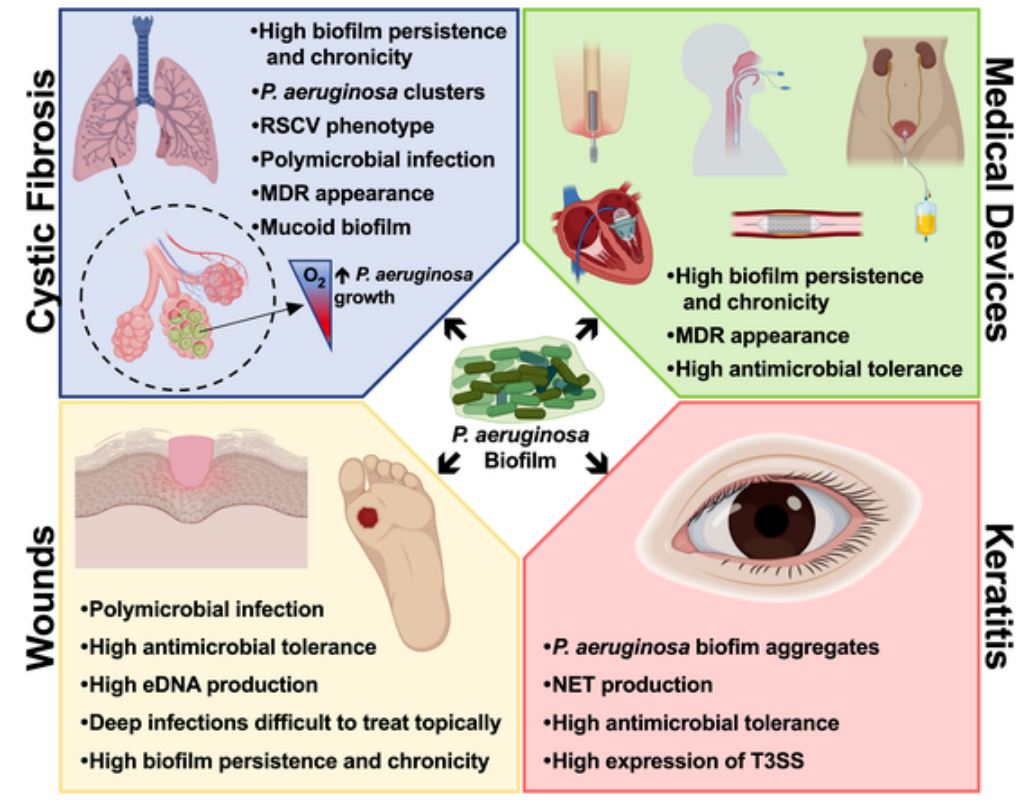
Fig 1. Main features of P. aeruginosa biofilms and the respective consequences on the infection progression.
One for all and all for one
Bacterial biofilms are formed by different species that colonize and grow together on human tissues, causing infections in the urinary tract, lung and kidney among others, and on surfaces of medical devices, as joint prostheses, heart valves and pacemakers, dental and breast implants, sutures and catheters. This broad presence gives an idea of the far-reaching of biofilm impact in the clinic.
The gregarious way of life where bacteria protect each other, makes it extremely difficult to fight against biofilms with classical approaches used for single bacterial infections. Bacteria produce extracellular substances that act as support and protection for the whole community. They have also the ability to communicate among them using a complex chemical system known as quorum sensing to coordinatively regulate the growth and establishment of the biofilm.
Taken together, biofilm characteristics make them more resistant to antibiotics and external stresses, and to patient’s defense responses. In some cases, to effectively treat a bacterial biofilm infection, the dose of antibiotic used must be multiplied by 1000 compared to a single infection, a concentration hard to achieve in patients without causing toxicity. Most of the time biofilm infections can only be treated by their removal, what makes it often unaffordable and lead to patients’ mental illness. Moreover, infections caused by multiple species are often associated with worse prognosis.
Present and future approaches to fight against bacterial biofilms
The complexity in treating bacterial biofilm’s infection with antibiotics and the emergence of antibiotic resistance led to the use of novel antimicrobial approaches with the objective to inhibit bacterium’s attachment to the surface; disrupt and disperse bacteria; inhibit the production of certain components that promote biofilm formation and increase antibiotic diffusion inside the biofilm. For instance, the use of quorum sensing inhibitors, biofilm-degrading compounds and antimicrobial peptides targeting specific P. aeruginosa molecules are the most used strategies. Bioengineered drug delivery systems are a good alternative to release therapeutic agents inside the biofilm, increasing specificity and reducing toxicity to the patient.
Historically, bacterial infections have been studied in vitro using individual species, without taking biofilms into account. However, we now know that biofilms are the predominant type of bacterial growth in nature and the cause of most human bacterial infections. “The actual challenge is to recreate models in the laboratory able to reproduce the biological and physiological characteristics of the bacterial biofilms”, concludes Maria del Mar Cendra, first author of the work.
Reference article: Maria del Mar Cendra and Eduard Torrents. Pseudomonas aeruginosa biofilms and their partners in crime. Biotechnology Advances (2021), 49, article 107734.





