About
We aim at understanding how physical forces and molecular control modules cooperate to drive biological function.
We develop new technologies to map and perturb the main physical properties that determine how cells and tissues grow, move, invade and remodel.
By combining this physical information with systematic molecular perturbations and computational models we explore the principles that govern the interplay between chemical and physical cues in living tissues.
We study how these principles are regulated in physiology and development, and how they are derailed in cancer and aging.
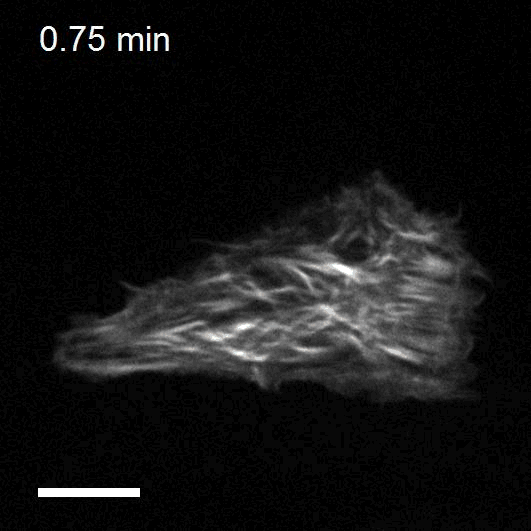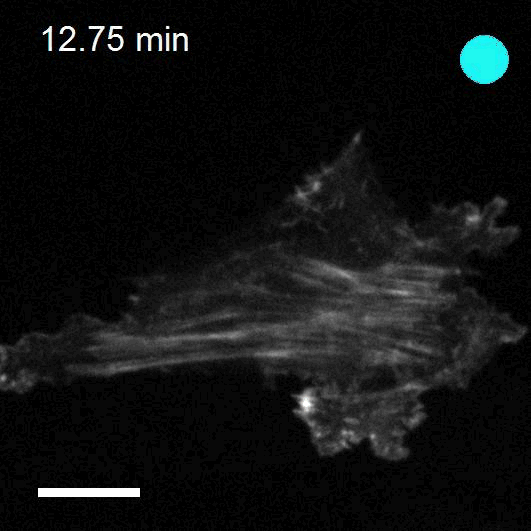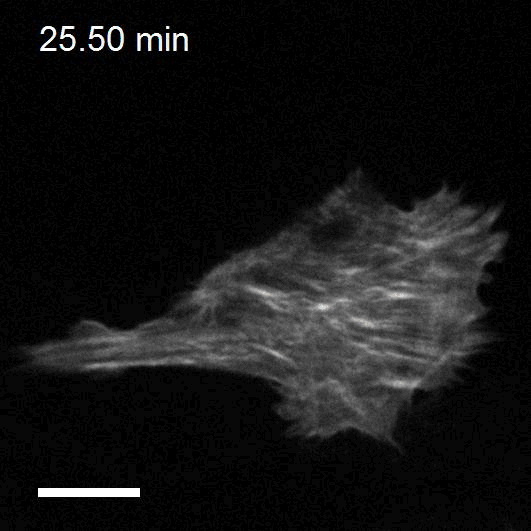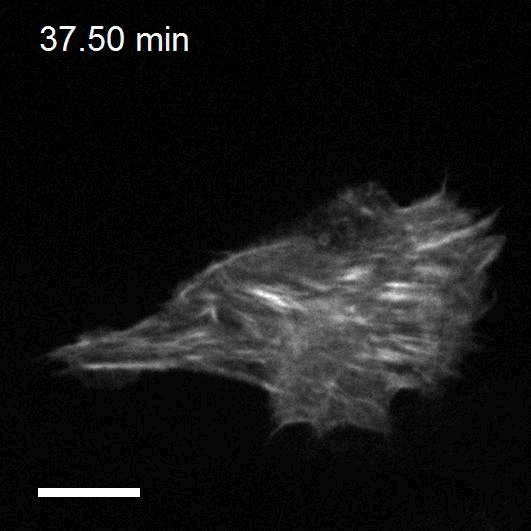
Making cellular forces visible
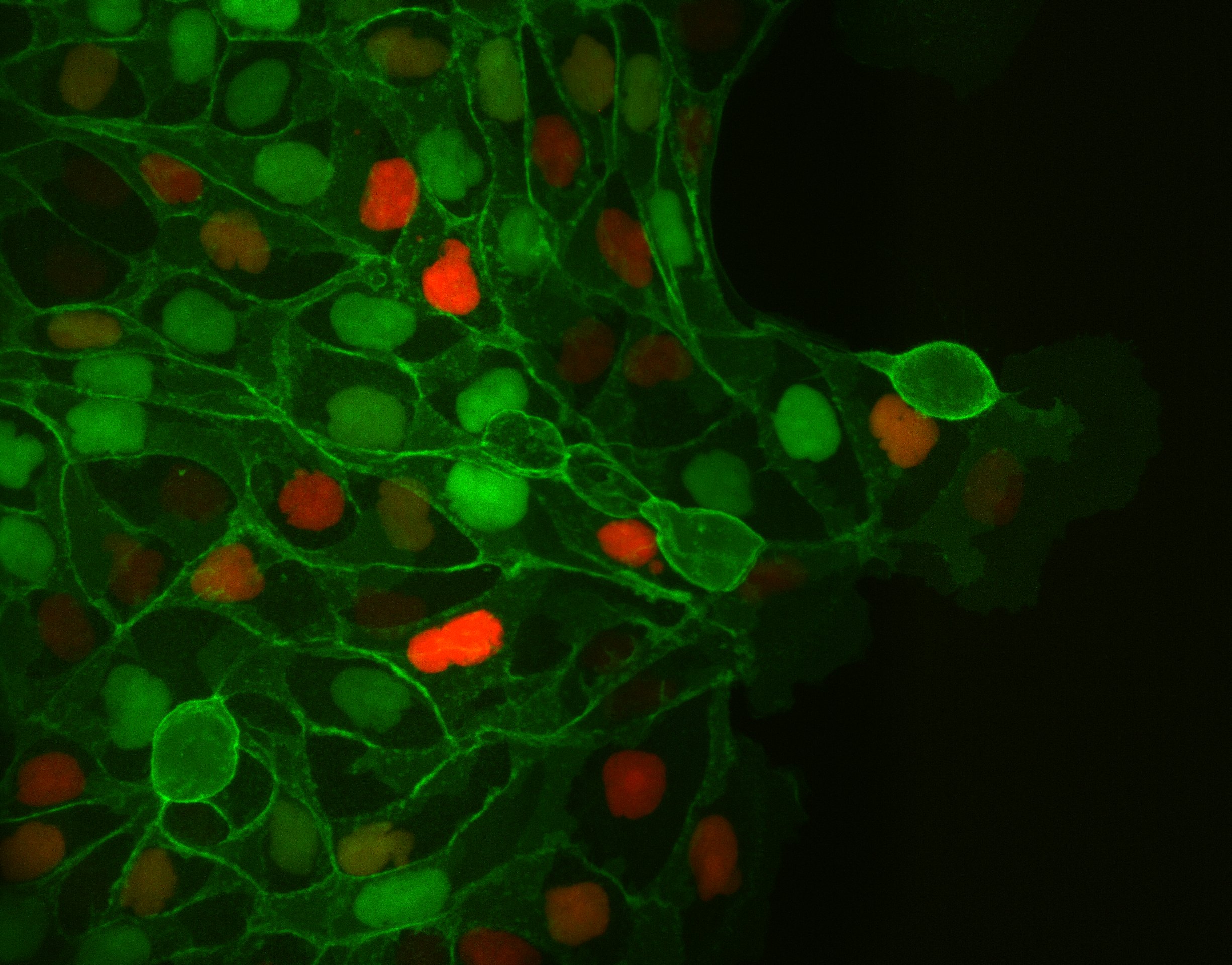
To study cell and tissue dynamics we develop new technologies to measure physical forces at the cell-cell and cell-matrix interface. By combining these technologies with computational analysis of cell shape and velocity we obtain a full experimental characterization of epithelial dynamics during tissue growth, wound healing and cancer cell invasion.
Tumour invasion by stromal forces
Cancer cell invasion and metastasis remain the leading cause of death in patients with cancer. Both processes are the result of a complex interaction between tumor cells and their microenvironment. One of our main lines of research is to study how tumours exploit the functions of non-cancer cells in their microenvironment to invade and metastasize. We focus on the interaction between epithelial cancer cells and Cancer Associated Fibroblasts (CAFs), the most abundant cell type in the tumour stroma.
Optogenetics to control cell mechanics
The recent development of optogenetic technologies offers promising possibilities to control signalling pathways with high spatiotemporal resolution. By expressing genetically encoded light-sensitive proteins, optogenetic technology enables the reversible perturbation of intracellular biochemistry with subcellular resolution. We have developed optogenetic tools based on controlling the activity of endogenous RhoA to upregulate or downregulate cell contractility and to control cell shape and mechanotransduction.
Collective durotaxis: a mechanism for cellular guidance by mechanical cues
Directed cell migration is one of the earliest observations in cell biology, dating back to the late XIX century. Also known as taxis, directed cell migration has been commonly associated with chemotaxis, i.e. the ability of a broad variety of cell types to migrate following gradients of chemical factors. We recently demonstrated a new mode of collective cell guidance by mechanical cues, called collective durotaxis. This new migration mode emerges only in cell collectives and, strikingly, does not require isolated cells to exhibit gradient sensing.
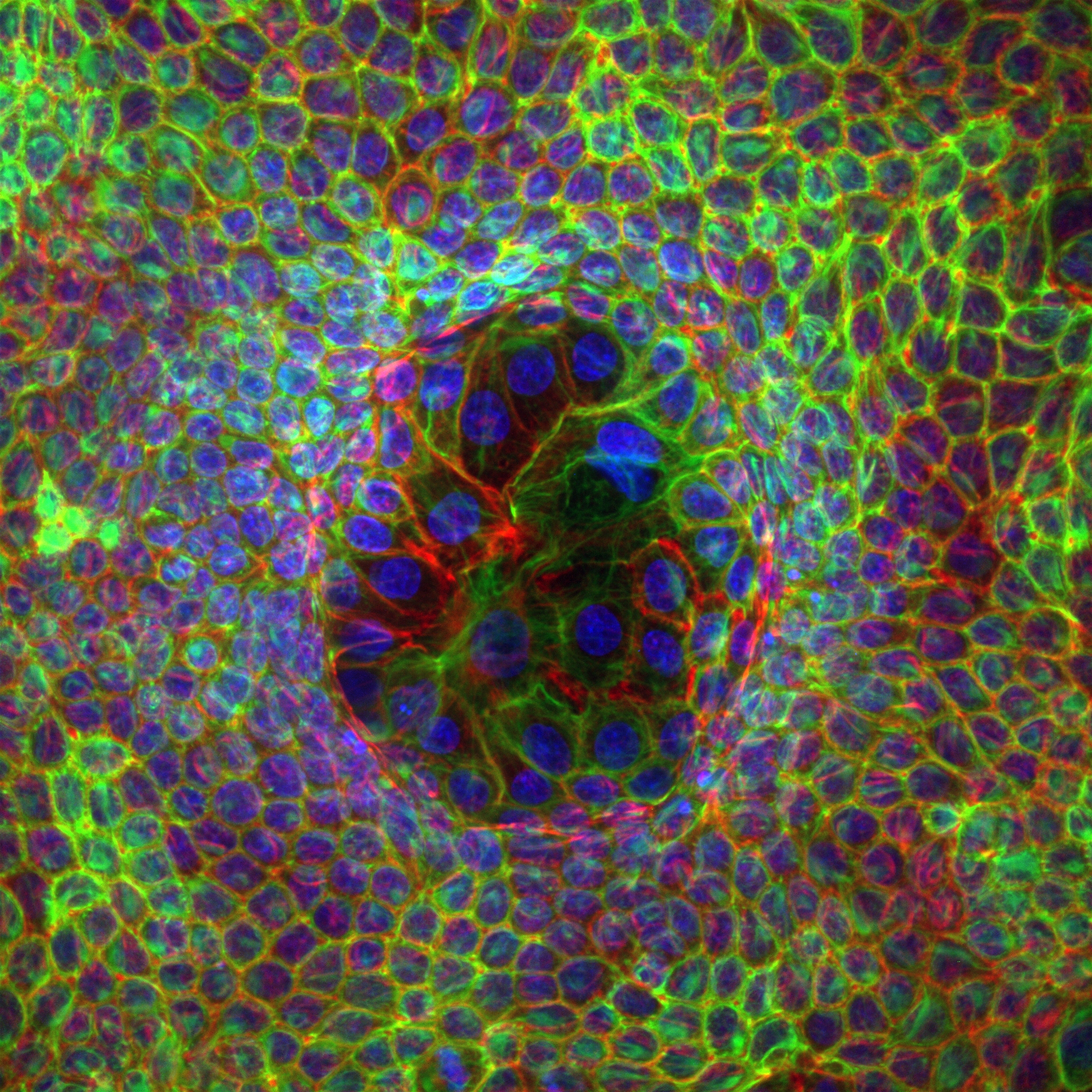
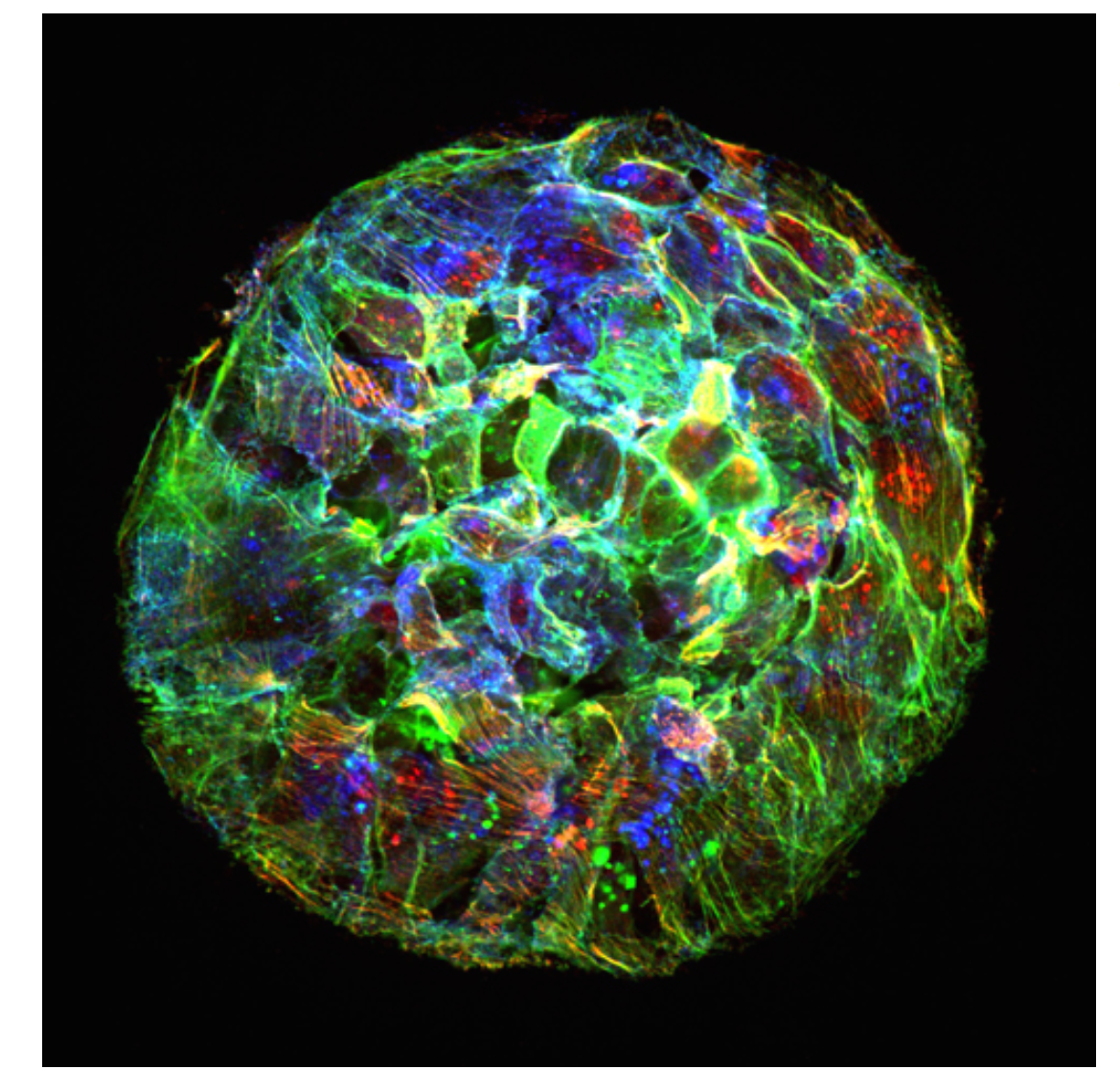
Organoid mechanobiology
Organoids are large multicellular structures that self-organize in vitro and maintain a similar organization and functionality than the organ from which they are derived. Organoids from many organs have now been obtained from embryonic stem cells, induced pluripotent stem cells and organ progenitors. We use intestinal and kidney organoids to study how epithelia adopt three-dimensional shapes that closely resemble their structure in vivo. We also use organoids grown from primary tumors to understand how epithelial structure and function are lost with disease progression.
Engineering epithelial shape and mechanics from the bottom up
We develop new approaches to engineer epithelia in 3D. Using these approaches, we study the principles that govern the emergence of tissue shape from the bottom up. We recently found that epithelial sheets can stretch up to four times their initial area without breaking, and that they are able to recover their initial size in a fully reversible way when unstretched. Surprisingly, some cells in the tissue barely stretch, while others become ‘superstretched’, increasing their area more than ten times. We call this phenomenon ‘active superelasticity’.
Staff
[br]
Projects
| NATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| mGRADIENTMecanobiología de la migración colectiva durante la haptotaxis y la durotaxis: aplicación a los organoides intestinales (2019-2022) | MICIU Generación Conocimiento: Proyectos I+D | Xavier Trepat |
| DYNAGELHidrogeles biocompatibles con rigidez dinámicamente ajustable para estudiar la mecanobiología de células y tejidos (2019-2022) | MICIU Retos investigación: Proyectos I+D | Raimon Sunyer |
| INTERNATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| EpiFold Engineering epithelial shape and mechanics: from synthetic morphogenesis to biohybrid devices (2021-2025) | European Commission, ERC-AdG | Xavier Trepat |
| The role of intermediate filaments in stress resistance in 3D epithelial structures (2021-2023) | Deutsche Forschungsgemeinschaft (DFG), Walter Benjamin-Programme | Tom Golde |
| Mechano·Control Mechanical control of biological function (2017-2022) | European Commission, FET Proactive | Xavier Trepat |
| Control of cell collective flows and tissue folding by means of surface patterns (2021-2022) | Human Frontier Science Program, HFSP Beca postdoctoral | Pau Guillamat |
| PRIVATELY-FUNDED PROJECTS | FINANCER | PI |
|---|---|---|
| Mech4Cancer · Enabling technologies to map nuclear mechanosensing: from organoids to tumors (2020-2023) | Obra Social La Caixa: Health Research Call | Xavier Trepat |
| T cell exclusion during cancer immune evasion and immunotherapy failure: cell types, transcriptional programs and biomechanics (2020-2023) | Fundació La Marató de TV3 | Xavier Trepat |
| Joint Programme Healthy Ageing | Obra Social La Caixa | Xavier Trepat |
| Understanding and measuring mechanical tumor properties to improve cancer diagnosis, treatment, and survival: Application to liquid biopsies (2017-2022) | Obra Social La Caixa | Xavier Trepat |
| FINISHED PROJECTS | FINANCER | PI |
|---|---|---|
| OPTOLEADER Optogenetic control of leader cell mechanobiology during collective cell migration (2019-2021) | European Commission, MARIE CURIE – IF | Leone Rossetti |
| MECHANOIDS Probing and controlling the three-dimensional organoid mechanobiology (2019-2021) | European Commission, MARIE CURIE – IF | Manuel Gómez |
| TensionControl Multiscale regulation of epithelial tension (2015-2020) | European Commission, ERC – CoG | Xavier Trepat |
| El mecanoma de la adhesión epitelial: mecanismos de detección, resistencia y transmisión de fuerzas intercelulares | MINECO, I+D-Investigación fundamental no orientada | Xavier Trepat |
| MICROGRADIENTPAGE Micro Gradient Polyacrylamide Gels for High Throughput Electrophoresis Analysis | European Commission, ERC-PoC | Xavier Trepat |
| GENESFORCEMOTION Physical Forces Driving Collective Cell Migration: from Genes to Mechanism | European Commission, ERC-StG | Xavier Trepat |
| CAMVAS Coordination and migration of cells during 3D Vasculogenesis (2014-2017) | European Commission, MARIE CURIE – IOF | Xavier Trepat |
| DUROTAXIS Mecanobiología de la durotaxis: de las células aisladas a los tejidos | MINECO, Proyectos I+D Excelencia | Xavier Trepat |
Publications
Check for more detailed information on the outputs of the Group at IBEC CRIS portal.
Publications list:
Equipment
- Soft Lithography
- Micro/Nano fabrication
- Cell stretching
- Live Confocal Microcopy
- Magnetic Tweezers
- Magnetic Twisting Cytometry
- Monolayer stress microscopy
- Traction microscopy
Collaborations
- Julien Colombelli / Eduard Batlle
Institute for Research in Biomedicine (IRB) Barcelona - Marino Arroyo
Universitat Politècnica de Catalunya, Barcelona - Guillaume Charras / Roberto Mayor
University College London, UK - Erik Sahai
Cancer Research, UK - Benoit Ladoux
Université Paris 7, France - Jim Butler & Jeff Fredberg
Harvard University, Boston - Danijela Vignjevic
Institut Curie, Paris - Jonel Trebicka
Department of Internal Medicine I, University Hospital Frankfurt - Eduard Batlle
Institute for Research in Biomedicine (IRB) Barcelona
News

IBEC successfully holds Hackathon for its new co-creation programme, ‘Open Dialogues’
Yesterday, the Institute for Bioengineering of Catalonia (IBEC) brought together around twenty artists and scientists for a co-creation hackathon as part of its new ‘Open Dialogues’ initiative. Following the event, two pairs of artists and scientists were selected to collaborate on developing two projects based on IBEC research over the next five months.

IBEC research takes third place in the 2025 Vanguardia de la Ciencia Awards
A study led by IBEC has won the 3rd Vanguardia de la Ciencia 2025 Prize. The research, led by Samuel Sánchez Ordoñez and with Meritxell Serra-Casablanca as lead author, proposes an innovative bladder cancer therapy based on nanorobots loaded with radiopharmaceuticals. These nanorobots are capable of moving around the bladder using urine as a source of energy.

Bioengineering for precision medicine at the 18th IBEC Symposium
The 18th annual IBEC Symposium focused on ‘Bioengineering for Precision Medicine’, which is one of IBEC’s key areas of application. The event was attended by nearly 300 people, including local and international researchers. The multidisciplinary environment provided experts from other centres and the IBEC community with the opportunity to present their projects and exchange knowledge.

IBEC and EMBL Barcelona co-organise a matchmaking workshop to explore synergies
Today, the Institute for Bioengineering of Catalonia (IBEC) and the European Molecular Biology Laboratory (EMBL) held a matchmaking workshop. The event brought together leading researchers from both centres to encourage the formation of new connections and promote scientific dialogue.
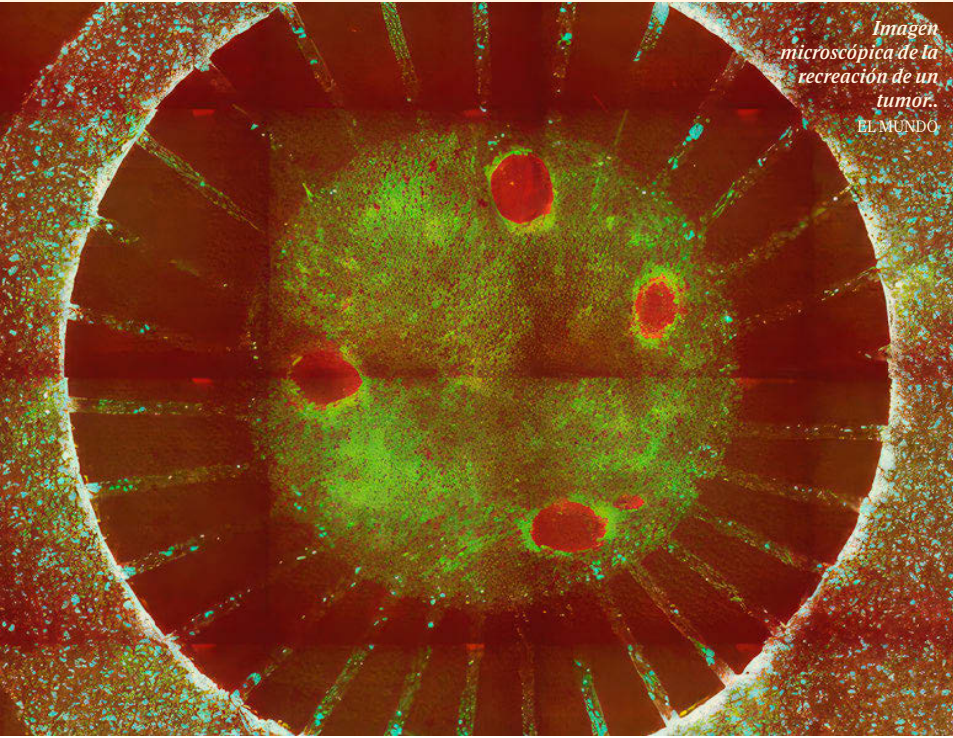
El Mundo: Un ‘gemelo’ de laboratorio para estudiar la eficacia de los tratamientos contra el cáncer
Científicos del IBEC, liderados por Xavier Trepat, han desarrollado MIRO, un dispositivo que recrea el entorno tumoral para estudiar su respuesta a tratamientos. Publicado en Nature Communications, permite analizar por … Read more

RTVE: Desarrollan un dispositivo para mejorar los resultados de tratamientos de inmunoterapia
Investigadores de l’Hospital del Mar y del IBEC están desarrollando un dispositivo para mejorar los resultados de los tratamientos de inmunoterapia contra el cáncer. Este dispositivo podría optimizar la eficacia … Read more
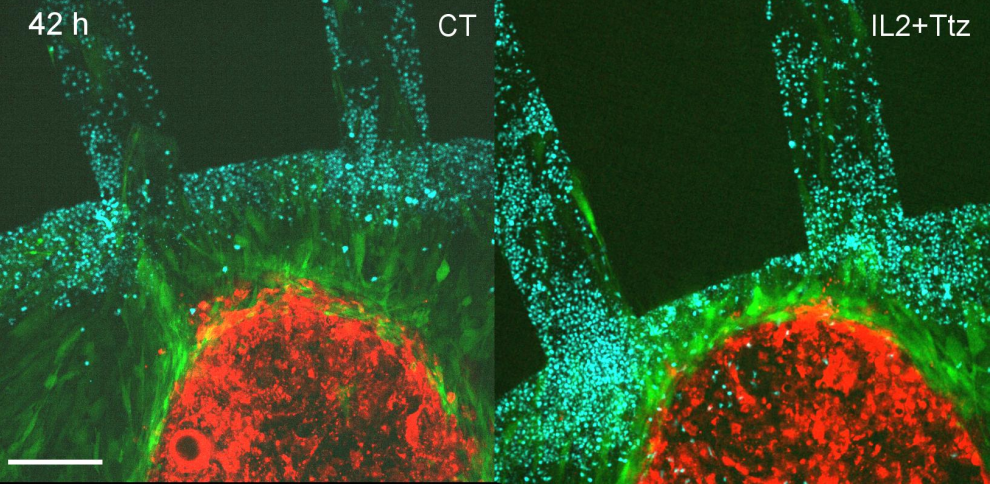
La Vanguardia: Investigadores catalanes recrean de forma fidedigna el ambiente tumoral para atacar el cáncer
Investigadores del IBEC y del Hospital del Mar han desarrollado MIRO, un dispositivo que recrea fielmente el entorno tumoral con células de pacientes. Esta innovadora tecnología permite estudiar la interacción … Read more

Researchers develop a device that replicates tumours to study the efficacy of immunotherapy treatments
The Micro Immune Response On chip (MIRO) allows tumours and their environment to be replicated in order to understand their response to treatment. The device, which has already been successfully tested on breast cancer samples, could be key to developing new treatments and determining the most appropriate therapy for each patient in a personalized way. The work, published in Nature Communications, is the result of a collaboration between the Institute for Bioengineering of Catalonia and the Research Institute of the Hospital del Mar.

Regió 7: Científica solsonina formada a Harvard: “A internet hi confirmes qualsevol mentida”
En una entrevista amb Regió7, la científica solsonina Janet van der Graaf reflexiona sobre la facilitat de trobar informació a internet que confirmi qualsevol afirmació, independentment de la seva veracitat. … Read more
Jobs
Postoctoral Researcher at the Integrative Cell and Tissue Dynamics Research Group
Ref: PR-XT // Deadline: 21/12/2025
Laboratory Technician at the Integrative Cell and Tissue Dynamics Research Group
Ref: LA-XT // Deadline: 24/11/2025
Research assistant at the Integrative Cell and Tissue Dynamics Research Group
Ref: RA-XT // Deadline: 17/10/2025
Research Training at the Integrative Cell and Tissue Dynamics Research Group
Ref: RT_XT // Deadline: 15/08/2025
Research assistant at the Integrative Cell and Tissue Dynamics Research Group
Ref: RA-XT // Deadline: 24/07/2025
Research Assistant at the Integrative Cell and Tissue Dynamics Research Group
Ref: RA_XT//Deadline: 5/11/2024
Postdoc at the Integrative Cell and Tissue Dynamics Research Group
Ref: XT-PD/Deadline: 18/12/2023
Research assistant at the Integrative Cell and Tissue Dynamics Research Group
Ref: RA_XT/Deadline: 15/06/2023


 ibecbarcelona.eu
ibecbarcelona.eu