About
We aim at understanding how physical forces and molecular control modules cooperate to drive biological function.
We develop new technologies to map and perturb the main physical properties that determine how cells and tissues grow, move, invade and remodel.
By combining this physical information with systematic molecular perturbations and computational models we explore the principles that govern the interplay between chemical and physical cues in living tissues.
We study how these principles are regulated in physiology and development, and how they are derailed in cancer and aging.
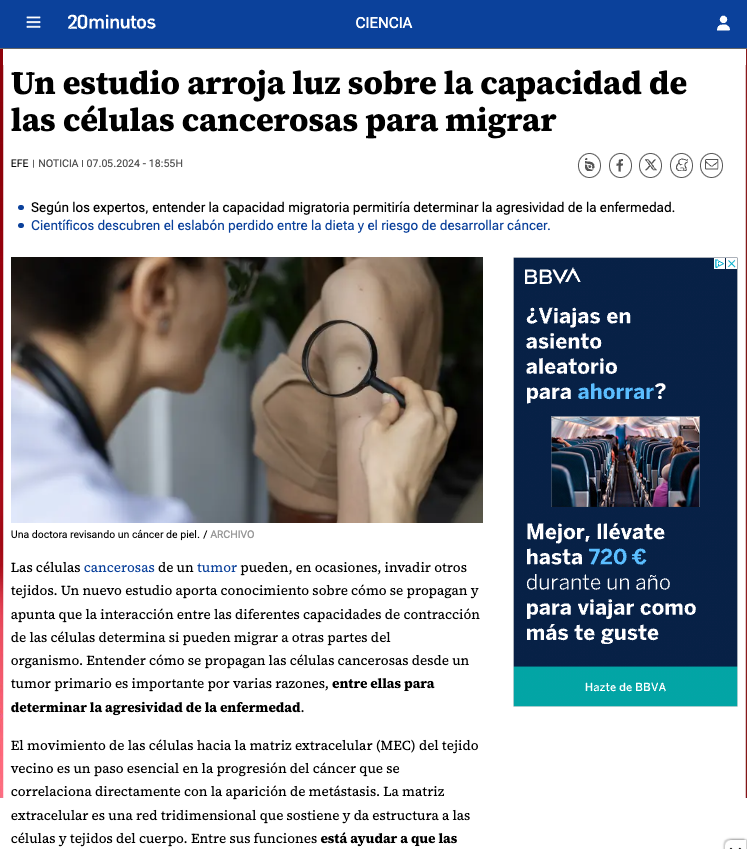
Making cellular forces visible
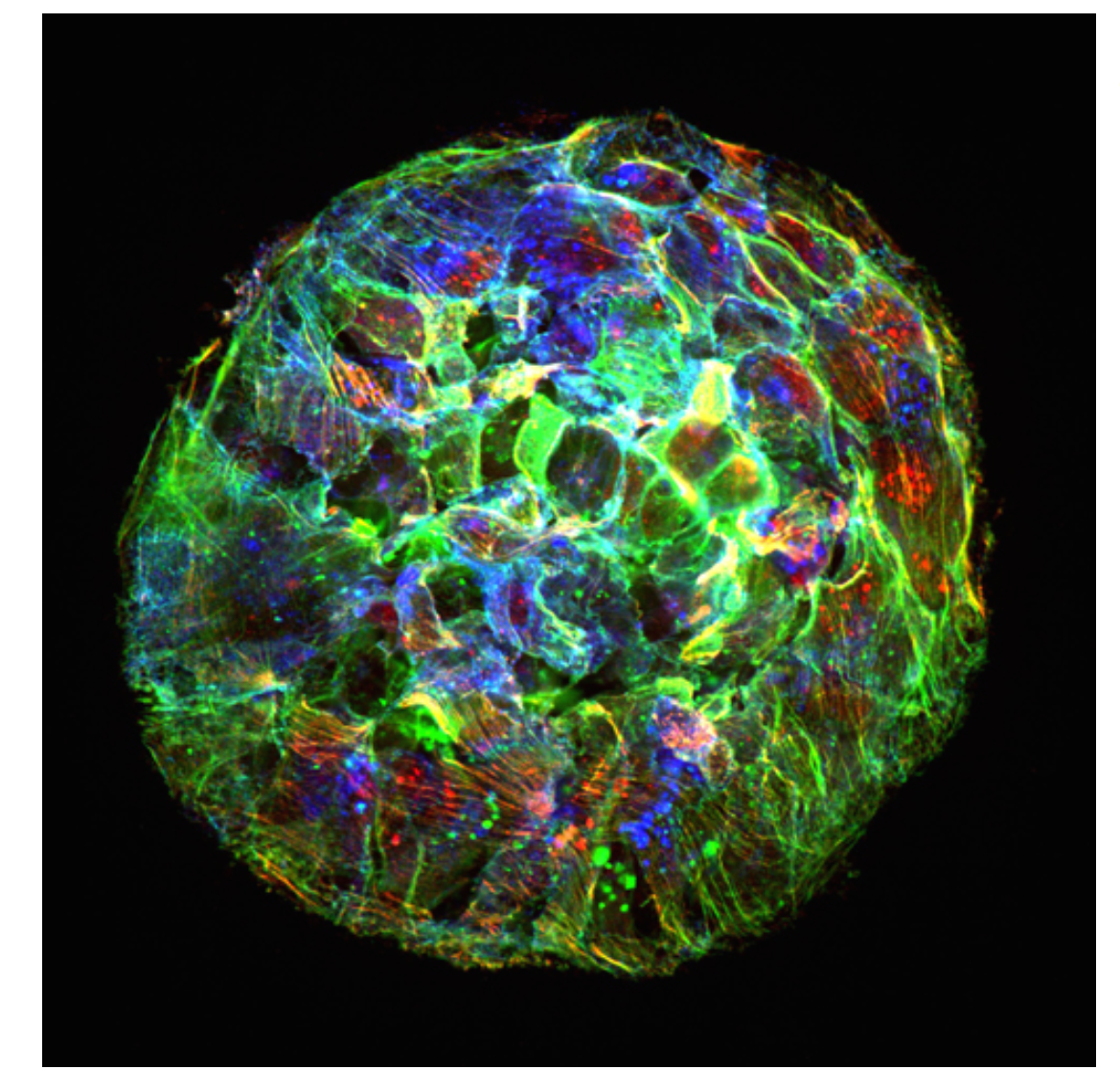
To study cell and tissue dynamics we develop new technologies to measure physical forces at the cell-cell and cell-matrix interface. By combining these technologies with computational analysis of cell shape and velocity we obtain a full experimental characterization of epithelial dynamics during tissue growth, wound healing and cancer cell invasion.
Tumour invasion by stromal forces
Cancer cell invasion and metastasis remain the leading cause of death in patients with cancer. Both processes are the result of a complex interaction between tumor cells and their microenvironment. One of our main lines of research is to study how tumours exploit the functions of non-cancer cells in their microenvironment to invade and metastasize. We focus on the interaction between epithelial cancer cells and Cancer Associated Fibroblasts (CAFs), the most abundant cell type in the tumour stroma.
Optogenetics to control cell mechanics
The recent development of optogenetic technologies offers promising possibilities to control signalling pathways with high spatiotemporal resolution. By expressing genetically encoded light-sensitive proteins, optogenetic technology enables the reversible perturbation of intracellular biochemistry with subcellular resolution. We have developed optogenetic tools based on controlling the activity of endogenous RhoA to upregulate or downregulate cell contractility and to control cell shape and mechanotransduction.
Collective durotaxis: a mechanism for cellular guidance by mechanical cues
Directed cell migration is one of the earliest observations in cell biology, dating back to the late XIX century. Also known as taxis, directed cell migration has been commonly associated with chemotaxis, i.e. the ability of a broad variety of cell types to migrate following gradients of chemical factors. We recently demonstrated a new mode of collective cell guidance by mechanical cues, called collective durotaxis. This new migration mode emerges only in cell collectives and, strikingly, does not require isolated cells to exhibit gradient sensing.
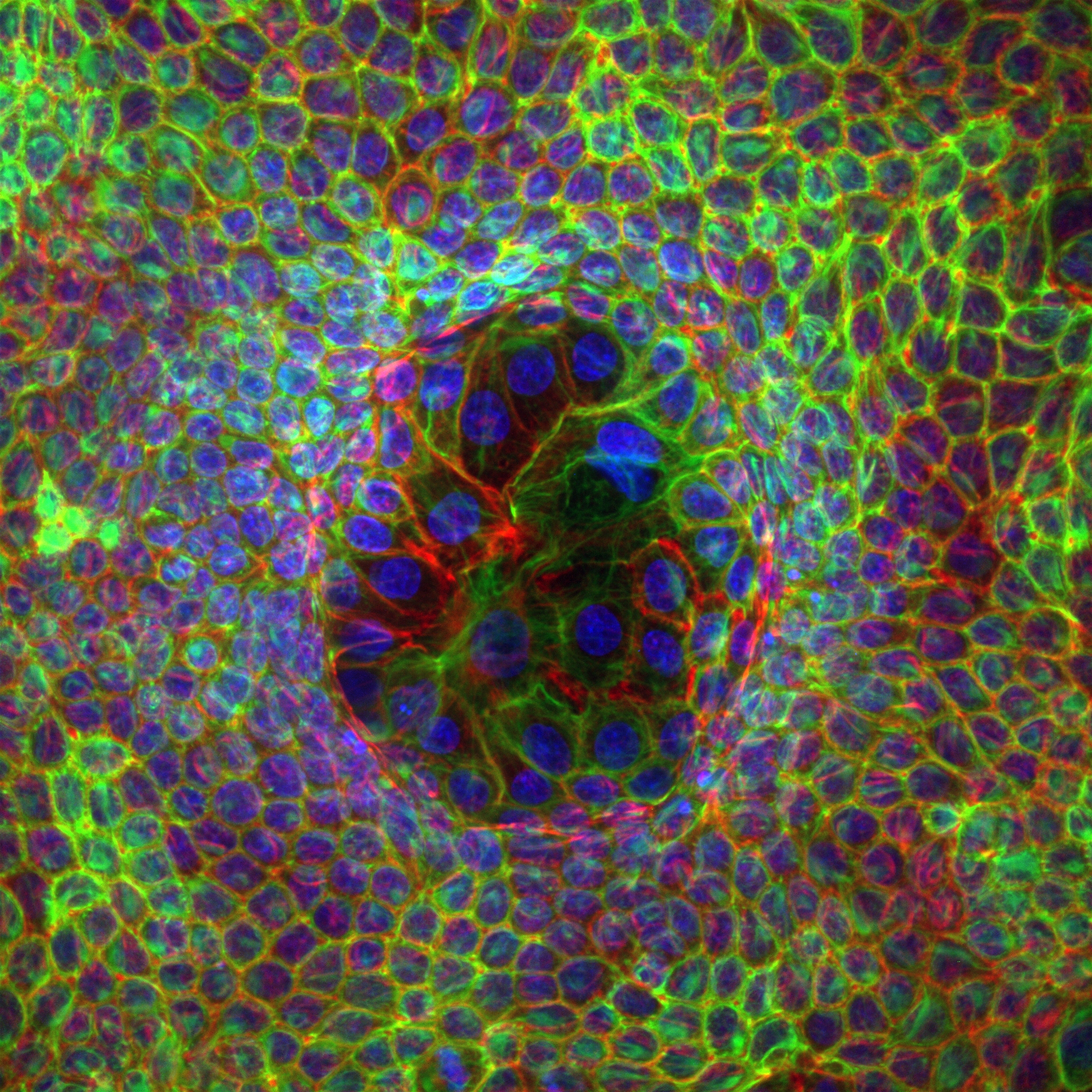
Organoid mechanobiology
Organoids are large multicellular structures that self-organize in vitro and maintain a similar organization and functionality than the organ from which they are derived. Organoids from many organs have now been obtained from embryonic stem cells, induced pluripotent stem cells and organ progenitors. We use intestinal and kidney organoids to study how epithelia adopt three-dimensional shapes that closely resemble their structure in vivo. We also use organoids grown from primary tumors to understand how epithelial structure and function are lost with disease progression.
Engineering epithelial shape and mechanics from the bottom up
We develop new approaches to engineer epithelia in 3D. Using these approaches, we study the principles that govern the emergence of tissue shape from the bottom up. We recently found that epithelial sheets can stretch up to four times their initial area without breaking, and that they are able to recover their initial size in a fully reversible way when unstretched. Surprisingly, some cells in the tissue barely stretch, while others become ‘superstretched’, increasing their area more than ten times. We call this phenomenon ‘active superelasticity’.
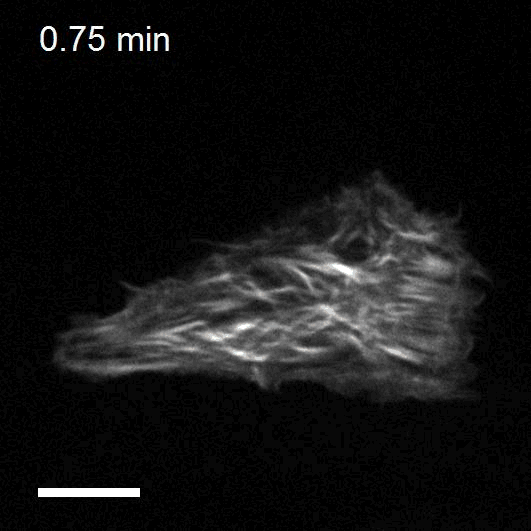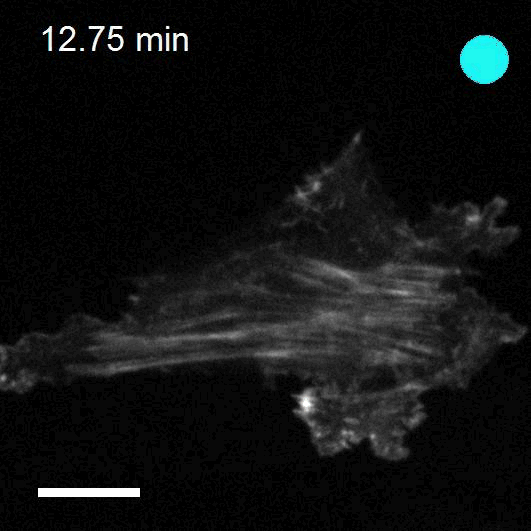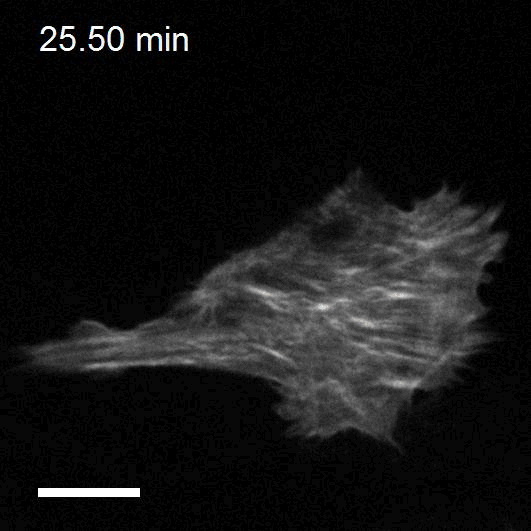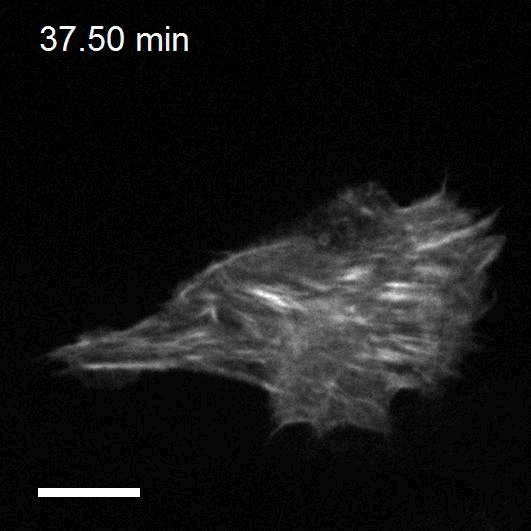
Staff
Xavier Trepat
Projects
| NATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| mGRADIENTMecanobiología de la migración colectiva durante la haptotaxis y la durotaxis: aplicación a los organoides intestinales (2019-2022) | MICIU Generación Conocimiento: Proyectos I+D | Xavier Trepat |
| DYNAGELHidrogeles biocompatibles con rigidez dinámicamente ajustable para estudiar la mecanobiología de células y tejidos (2019-2022) | MICIU Retos investigación: Proyectos I+D | Raimon Sunyer |
| INTERNATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| EpiFold Engineering epithelial shape and mechanics: from synthetic morphogenesis to biohybrid devices (2021-2025) | European Commission, ERC-AdG | Xavier Trepat |
| The role of intermediate filaments in stress resistance in 3D epithelial structures (2021-2023) | Deutsche Forschungsgemeinschaft (DFG), Walter Benjamin-Programme | Tom Golde |
| Mechano·Control Mechanical control of biological function (2017-2022) | European Commission, FET Proactive | Xavier Trepat |
| Control of cell collective flows and tissue folding by means of surface patterns (2021-2022) | Human Frontier Science Program, HFSP Beca postdoctoral | Pau Guillamat |
| PRIVATELY-FUNDED PROJECTS | FINANCER | PI |
|---|---|---|
| Mech4Cancer · Enabling technologies to map nuclear mechanosensing: from organoids to tumors (2020-2023) | Obra Social La Caixa: Health Research Call | Xavier Trepat |
| T cell exclusion during cancer immune evasion and immunotherapy failure: cell types, transcriptional programs and biomechanics (2020-2023) | Fundació La Marató de TV3 | Xavier Trepat |
| Joint Programme Healthy Ageing | Obra Social La Caixa | Xavier Trepat |
| Understanding and measuring mechanical tumor properties to improve cancer diagnosis, treatment, and survival: Application to liquid biopsies (2017-2022) | Obra Social La Caixa | Xavier Trepat |
| FINISHED PROJECTS | FINANCER | PI |
|---|---|---|
| OPTOLEADER Optogenetic control of leader cell mechanobiology during collective cell migration (2019-2021) | European Commission, MARIE CURIE – IF | Leone Rossetti |
| MECHANOIDS Probing and controlling the three-dimensional organoid mechanobiology (2019-2021) | European Commission, MARIE CURIE – IF | Manuel Gómez |
| TensionControl Multiscale regulation of epithelial tension (2015-2020) | European Commission, ERC – CoG | Xavier Trepat |
| El mecanoma de la adhesión epitelial: mecanismos de detección, resistencia y transmisión de fuerzas intercelulares | MINECO, I+D-Investigación fundamental no orientada | Xavier Trepat |
| MICROGRADIENTPAGE Micro Gradient Polyacrylamide Gels for High Throughput Electrophoresis Analysis | European Commission, ERC-PoC | Xavier Trepat |
| GENESFORCEMOTION Physical Forces Driving Collective Cell Migration: from Genes to Mechanism | European Commission, ERC-StG | Xavier Trepat |
| CAMVAS Coordination and migration of cells during 3D Vasculogenesis (2014-2017) | European Commission, MARIE CURIE – IOF | Xavier Trepat |
| DUROTAXIS Mecanobiología de la durotaxis: de las células aisladas a los tejidos | MINECO, Proyectos I+D Excelencia | Xavier Trepat |
Publications
Equipment
- Soft Lithography
- Micro/Nano fabrication
- Cell stretching
- Live Confocal Microcopy
- Magnetic Tweezers
- Magnetic Twisting Cytometry
- Monolayer stress microscopy
- Traction microscopy
Collaborations
- Julien Colombelli / Eduard Batlle
Institute for Research in Biomedicine (IRB) Barcelona - Marino Arroyo
Universitat Politècnica de Catalunya, Barcelona - Guillaume Charras / Roberto Mayor
University College London, UK - Erik Sahai
Cancer Research, UK - Benoit Ladoux
Université Paris 7, France - Jim Butler & Jeff Fredberg
Harvard University, Boston - Danijela Vignjevic
Institut Curie, Paris - Jonel Trebicka
Department of Internal Medicine I, University Hospital Frankfurt - Eduard Batlle
Institute for Research in Biomedicine (IRB) Barcelona
News

Xavier Trepat Wins the Lilly Foundation Award for Biomedical Research 2024
IBEC ICREA Professor Xavier Trepat has been awarded the Lilly Foundation Award in the category of preclinical research. With this accolade, the Fundación Lilly acknowledges Trepat’s work, distinguished by the application of techniques and concepts from physics to the health sciences.

The interdisciplinarity of IBEC showcased in the opinion section of Nature Physics
Teresa Sanchis and Xavier Trepat, members of IBEC, have authored opinion pieces in the ‘World View’ section of Nature Physics. Both articles underscore IBEC’s dedication to scientific excellence, the promotion of diversity, and the cultivation of an environment conducive to collaboration. They also highlight the relevant role of IBEC at the international level in multidisciplinary research.

Bioengineering Becomes Art at the Antoni Tàpies Museum
An installation featured in the exhibition “A=A, B=B” at the Antoni Tàpies Foundation will provide live access to the laboratories of IBEC. A window from which visitors will have the opportunity to see how researchers carry out their research projects, as if it were a genuine performance of scientific innovation.
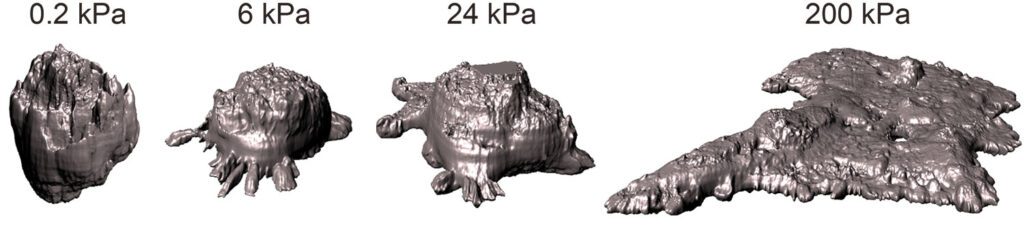
Preventing the tissue’s response to stiffness may be key to slowing the progression of breast tumors
A study led by the Institute of Bioengineering of Catalonia demonstrates that laminin, a protein present in breast tissues, prevents the effects of stiffening, protecting cells against tumor growth. While the mechanism has been demonstrated in vitro, persuasive indications suggest its potential applicability in vivo, as observed in patient samples.

The internal clock of our cells is influenced by mechanical forces
IBEC researchers uncover how mechanical forces disrupt the circadian clock in cells, the mechanism governing daily physiological changes. The finding may help to better understand aging and specific diseases, like … Read more

Daniel Navajas: 30 years dedicated to mechanobiology
Last May 5, on the occasion of Professor Daniel Navajas’ retirement, the IBEC held the symposium Before Mechanobiology had a name. The event paid tribute to the IBEC researcher’s exciting … Read more
Cancer cells move to rigid environments like droplets
Researchers from the Institute of Bioengineering of Catalonia (IBEC) and the University of Barcelona (UB) uncovered a similarity between liquid droplets and cell groups, revealing that surface tension helps cells to … Read more

Mechanosensing in the media
IBEC researchers led by Pere Roca-Cusachs and Xavier Trepat appears in the media for a study that opens doors for new research into cancer therapies and diagnostics.

EMBL-IBEC Conference in the media
The EMBL-IBEC Conference, a three-day conference organized by the Institute for Bioengineering of Catalonia (IBEC) and the European Molecular Biology Laboratory (EMBL), is featured in the journal ARA where international experts discussed how to reproduce human diseases in living systems generated in the laboratory.


 ibecbarcelona.eu
ibecbarcelona.eu