IBEC and ISGlobal researchers led a study that points towards protein aggregation as a possible target to find new ways to reduce the viability of Plasmodium falciparum, the main causing agent of malaria. By inducing protein aggregation, they observed considerable disorders in protein homeostasis and a significant reduction in parasite growth. The results position protein aggregation control as a promising target for antimalarial therapies.
Malaria remains a persistent public health burden, with Plasmodium falciparum causing the most dangerous form of malaria and the most lethal infections. Its capacity to develop resistance to current treatments underlines the urgency of identifying new molecular targets.
In this context, a recent study led by the Nanomalaria research group from the Institute for Biengineering of Barcelona (IBEC) and the Barcelona Institute for Global Health (ISGlobal), has uncovered a potential vulnerability in P. falciparum by inducing protein aggregation within the parasite. Researchers induced the overexpression of a specific segment of an intrinsically disordered protein, significantly affecting parasite growth. The work, published in the journal Frontiers in Cellular and Infection Microbiology, may open the door for novel antimalarial strategies targeting the parasite’s internal protein folding machinery.
Uncovering a Potential Achilles’ Heel in Malaria Parasites
Central to this investigation is the concept of protein aggregation, which refers to the accumulation of misfolded or unfolded proteins into insoluble clusters. In humans, this process is associated with several neurodegenerative diseases, including Alzheimer’s and Parkinson’s, where aggregated proteins disrupt cellular functions and lead to cell death. Protein aggregation is typically counteracted by molecular chaperones and proteasomal systems that maintain proteostasis—an essential cellular balance of protein synthesis, folding, and degradation.
In P. falciparum, however, the scenario is particularly intriguing. On the one side, the parasite’s encodes a robust proteostasis network adapted to survive the intense metabolic changes and stress conditions it encounters during its life cycle, both inside its vector, mosquitoes from the genus Anopheles, and the human host.
By overexpressing a disordered segment of PfUT in P. falciparum, we disrupted this fragile balance, triggering proteotoxic stress and leading to reduced parasite growth.
Yunuen Avalos-Padilla
However, and contradictorily, proteins of P. falciparum are highly prone to aggregation. Despite its relatively small genome and efficient proteome, the high propensity to protein aggregation in this organism may reflect a fine-tuned evolutionary trade-off between functionality and instability. This property may confer the parasite specific adaptive advantages such as aiding in protein protection or facilitating the formation of stress-related aggregates that help it survive in hostile environments, such as fever in human host and oxidative stress in infected cells. In this sense, it possesses a relatively high proportion of proteins with intrinsically disordered regions —such as the ubiquitin-protein ligase, PfUT— which are generally more prone to misfolding and aggregation under stress.
This complex proteomic scenario led the researchers to explore whether an induced increase of a highly abundant and aggregation-prone protein like PfUT could shift the parasite’s proteome towards an aggregation state surpassing its proteostasis control machinery, leading to a decrease in its viability.
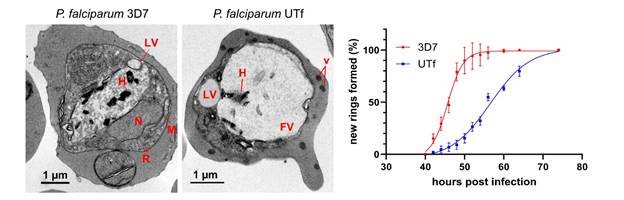
“By overexpressing a disordered segment of PfUT in P. falciparum, we disrupted this fragile balance, triggering proteotoxic stress and leading to reduced parasite growth. However, despite the observed alterations in proteostasis, the parasites were not killed by the increased aggregation of this particular protein, showing their capacity to control a highly aggregation-prone proteome”, explains Yunuen Avalos-Padilla, first author of the work. In other words, by overwhelming the parasite’s proteostasis system, researchers were able to compromise its survival, but Plasmodium could eventually recover.
This study not only highlights a key vulnerability in Plasmodium falciparum‘s internal protein management system but also positions protein aggregation control as a promising target for antimalarial intervention.
Xavier Fernández-Busquets
This discovery identifies a novel and potentially exploitable aspect of the parasite’s biology: its susceptibility to disruptions in protein aggregation control. Therapeutic strategies that amplify protein misfolding of certain key proteins or block the parasite’s ability to respond to aggregation could therefore offer a powerful new line of attack.
In words of Fernàndez-Busquets, “this study not only highlights a key vulnerability in Plasmodium falciparum‘s internal protein management system but also positions protein aggregation control as a promising target for antimalarial intervention. By deepening our understanding of the parasite’s proteostasis mechanisms, we may unlock new avenues for combating one of the world’s most persistent infectious diseases”.
Nonetheless, moving from laboratory findings to clinical application requires further work. Future studies will need to elucidate the precise molecular interactions involved and determine whether similar vulnerabilities exist in different life stages of the parasite. Additionally, any compounds developed to exploit this mechanism must be carefully assessed to ensure selectivity and safety.
Referenced article:
Yunuen Avalos-Padilla, Inés Bouzón-Arnáiz, Miriam Ramírez, Claudia Camarero-Hoyos, Marc Orozco-Quer, Elsa M. Arce, Diego Muñoz-Torrero and Xavier Fernàndez-Busquets. Overexpression in Plasmodium falciparum of an intrinsically disordered protein segment of PfUT impairs the parasite’s proteostasis and reduces its growth rate. Front. Cell. Infect. Microbiol. Volume 15 – 2025. doi: 10.3389/fcimb.2025.1565814





