This work, a breakthrough in biomolecular engineering, was led by Stefano Angioletti-Uberti at the Imperial College London and Institute for Bioengineering of Catalonia (IBEC) group leader Giuseppe Battaglia.
A growing area of interest in bioengineering is focused on how biomolecular binding observed in nature can be synthetically mimicked to aid drug delivery. If we could create a drug carrier that specifically binds to diseased cells and pathogens without harming healthy cells, we could develop highly targeted therapies with far fewer side effects. Now, thanks to a new study co-authored by ICREA Research Professor and IBEC Leader of IBEC’s Molecular Bionics Group—we are one step closer to reaching this goal. In this important work, recently published in the prestigious scientific journal Nature Communications, the authors describe how they achieved what they dub “range selectivity” in synthetic biomolecular binding. Researchers from ten centres contributed to this study, including Beijing University of Chemical Technology and Imperial College London.
Mimicking nature
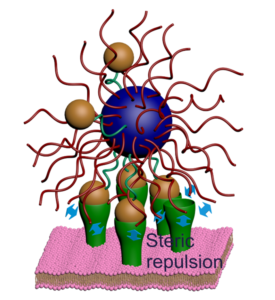
Ligand-receptor binding is the process through which cells receive signals from the external environment. Many biomolecules are multivalent, meaning that multiple ligands on one molecule attach to multiple receptors on another simultaneously. As a result, such molecules bind with exquisite selectivity. Successfully mimicking this would open up a host of new possibilities in biomedicine, such as the development of nano-sized drug carriers that only release their therapeutic load when they attach to specific diseased cells. However, such selective precision remains an unmet goal in bioengineering.
Now, in this new publication, the researchers describe how they built nanoparticles that only bind to receptors when receptor density is within a precise range, but not below nor above—a phenomenon that they dub “range selectivity”. They point out that this occurs due to a balance of attractive and repulsive forces at the molecular level. Past a certain threshold, the repulsive forces outweigh the attractive forces, and so binding is far less likely to take place.
Using statistical mechanical modelling, they showed that certain parameters could be exploited to tune the upper and lower limit of the selectivity range. Thus, this study brings us one step closer to achieving synthetic nanoparticles that exclusively bind to target receptors.
Furthermore, it offers an important insight that could change the way that future studies in this field are conducted: increasing the number of receptors does not necessarily increase the probability of binding, and in fact may cause it to significantly decrease.
Towards targeted drug delivery
This work not only paves the way for more highly targeted drug delivery with fewer side effects due to off-target binding, it also offers clues that could potentially explain why tumours evade attack from the immune system. Specifically, cancer cells usually over-express receptors which are thus present at a very high density on their surface—potentially outside of the binding range of immune cells.
The authors conclude that more research should be done to better understand how different parameters can be exploited to finetune range selectivity and build highly effective drug carriers.
This important study brings us one step closer to achieving precision targeting down to the single cell population or even personalised to the individual patient
Beppe Battaglia
Reference article:
Liu, M., Apriceno, A., Sipin, M. et al. Combinatorial entropy behaviour leads to range selective binding in ligand-receptor interactions. Nat Commun 11, 4836 (2020).





