ABOUT
Mastering communication between synthetic or hybrid materials with tissues is one of the grand challenges of contemporary biomedical systems that our society demands.
We want to uncover design rules to develop materials capable of communicating with living matter —pathogens, cells, tissues— and directing its behavior in a self-regulated manner to enable new biomaterials, therapeutics, and medical devices.
Biosensors fail to accurate diagnose diseases. Orthopedic implants cause inflammation and often rejection. Bacteria colonize wound dressings, catheters, and implants with devastating outcomes. Bacteria have grown resistant to many therapies. The surface of oxygenator membranes activate coagulation posing serious risks of thrombosis and stroke. These and many more examples have in common that the interface of the synthetic material is not sufficiently compatible resulting in deleterious signals to the living counterpart.
Nature blueprints are our inspiration
Like a master engineer, nature assembles its building blocks, molecules, in several levels of hierarchy from where function emerge. All the information necessary for this assembly is encoded in the molecular structure. We are inspired by natures’ and use its design principles to create materials that seamlessly interface with living matter to develop new paradigms for biomedicine.
RESEARCH LINES
MOLECULAR ENGINEERING
We are excited about using nature’s blueprints to make new molecules like “Lego” pieces that assemble into structures with totally new functions. Our synthetic palette is built from various types of advanced controlled radical polymerizations, click ligations, and supramolecular chemistry. We like to focus on water-soluble and amphiphilic molecules because of the unique aspects of the assembly in aqueous environments. Our box of toys includes multiblock copolymers, combs, stars, brushes, protein-polymer hybrids, dendrimers, and arborescent polymers.
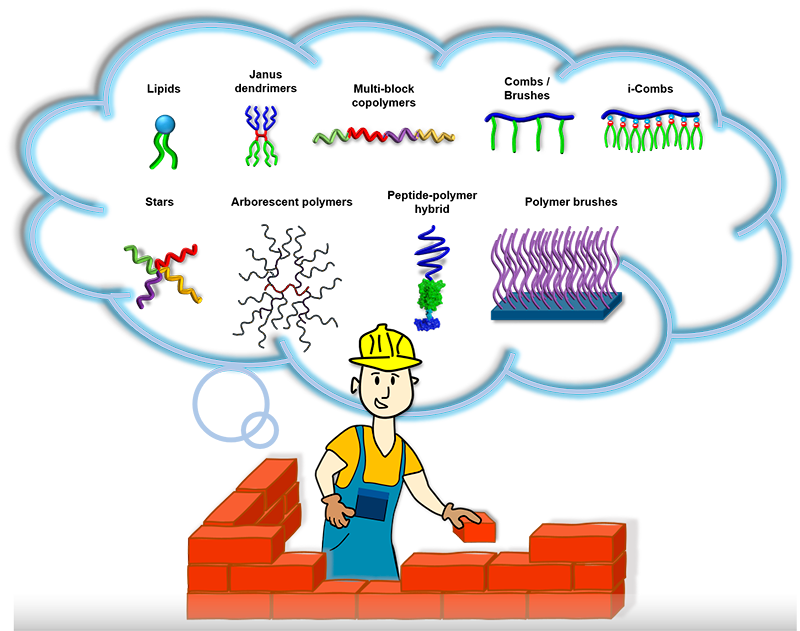
Macromolecular engineering: We synthesize macromolecular building blocks and program in their molecular structure their assembly and function
STEALTH AND ADAPTIVE BIOINTERFACES
Our goal is to develop adaptive coatings that interact and direct the biological surrounding in a self-regulated manner and their translation to medical and diagnostic applications. A cornerstone for this goal is to have selectivity, which means to be invisible to unwanted interactions while enhancing the specific ones. We are specialists in building “cloaks of invisibility”. These are nanoscale coatings that protect the surface against molecular fouling as well as bacterial colonization. They are also very important because they conceal the presence of synthetic materials.
But invisibility is not enough, so we decorate the surfaces with biomolecules and create feedback loops with the surrounding.
Examples:
- Optical affinity biosensors for the early diagnosis of (rare) diseases.
- Coatings that can sense the state of blood and selectively interfere with coagulation but just locally without affecting global hemostasis.
- Kill&Repel coatings that simultaneously prevent cellular adhesion and kill bacteria using mechanisms are selective and do not generate the emergence of resistance.
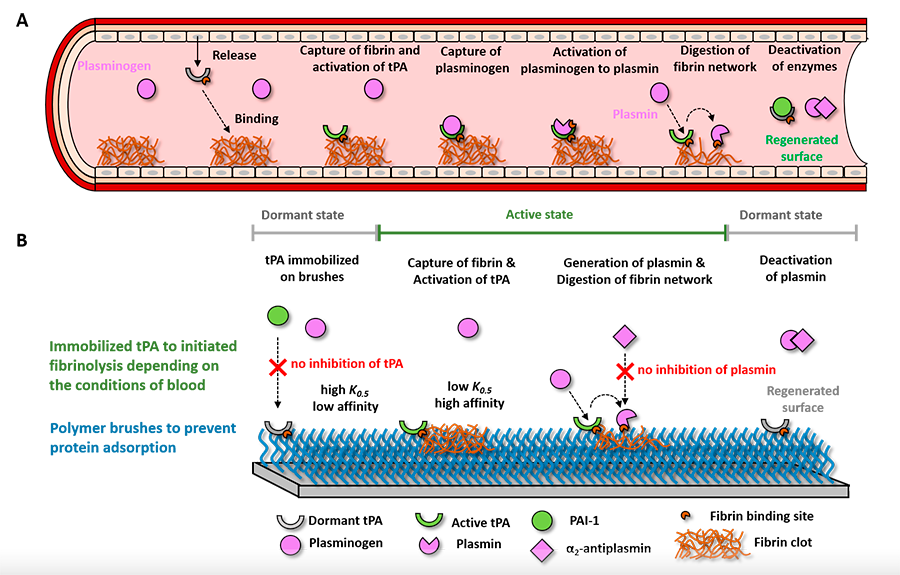
(A) Nature’s way to control the presence of clots inspires us to develop (B) adaptive hemocompatible coatings that in normal conditions are dormant and invisible to blood but that the presence of a dangerous clot causes its activation. In an active state, the coating directs blood to digest the clot thereby protecting the surface of the material.
Video 1. A macroscopic clot being digested by a fibrinolytic coating. DOI: 10.1021/acsami.1c01079

Kill&Repel coatings. The nanoscale coating selectively kills bacteria using the same weapons as bacteriophages and prohibits the adsorption of the debris of the dead bacteria. (DOI: 10.1002/adfm.202106656)
CELL MEMBRANE MIMICS AND PROTOCELLULAR SYSTEMS
We aim at building “quasi-living” synthetic cells (SynCells) by assembling non-living modules into micro-compartments displaying similar functionality and adaptivity as found in natural cells. This research holds promise to unveil some of the most daunting questions related to the origin of life, the transition from inanimate to living, and the emergence of diseases. We are not constrained to simply copy the current life forms which enable us to engineer synthetic cells with biologically inspired but augmented or even completely new functions to open new horizons for biomedicine, sensing, and therapeutics.
We are developing new concepts for cell membrane from non-natural amphiphiles with unprecedented mimicry to natural membranes but enhanced stability and which can be programmed to incorporate natural components of the cell membrane such as lipids, glycans, transmembrane proteins, etc. We enhance their livingness by hijacking functions of natural cells. For example by fusing the SynCells with bacteria or eukaryotic cells we integrate part of the complex molecular makeup of their membrane or by integrating active cell machinery, such as the divisome. We also investigate the introduction of superselectivity at the cell membrane to enable SynCells to recognize specific cells and actuate them. In particular, we are developing Synthetic Phagocytic Cells capable of engulfing and destroying pathogenic viruses and bacteria.
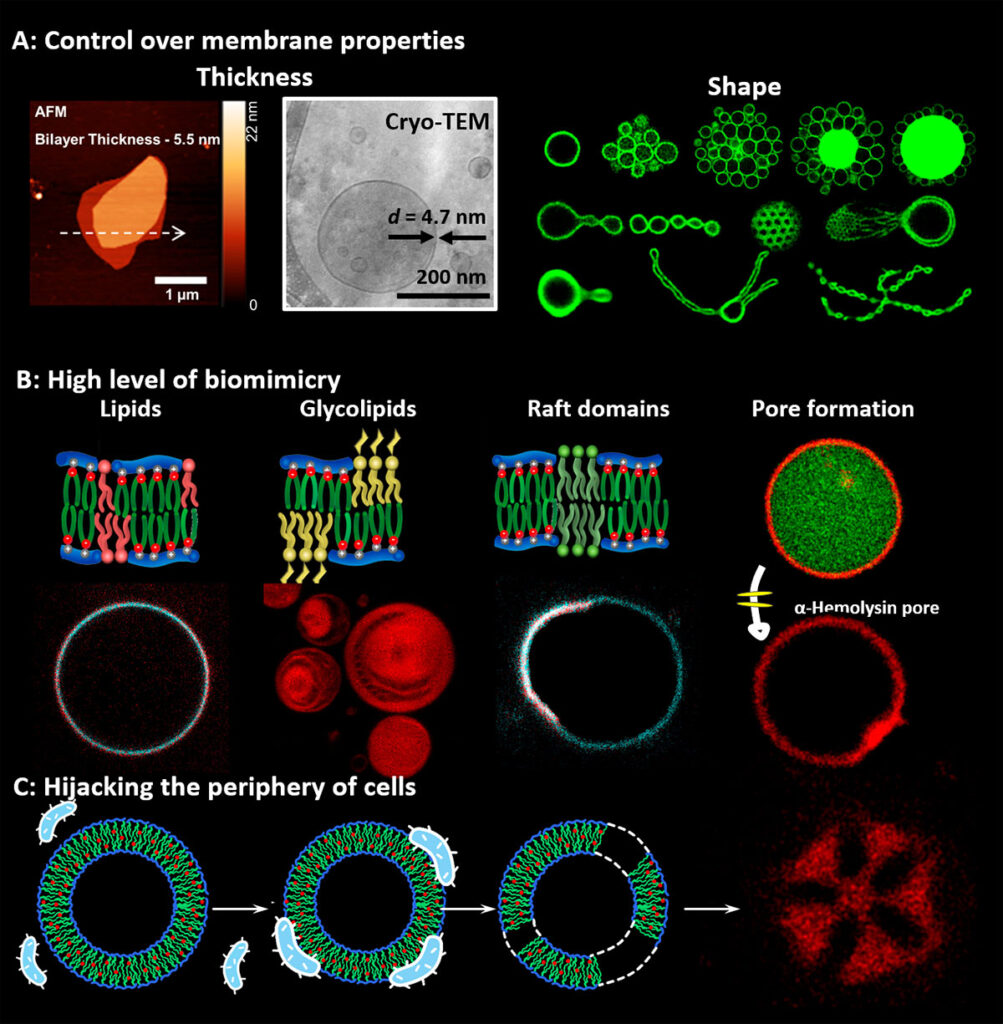
Examples of synthetic biomembranes. (A) We build vesicles from Janus dendrimers and amphiphilic comb polymers that accurately mimic the thickness, flexibility, and lateral mobility of natural membranes and to which we can program their shape. (B) The high biomimicry enables the incorporate functional lipids, glycolipids, and transmembrane proteins and to (C) form fuse with living cells to form hybrids of bacteria and synthetic cells.
TRANSLATION
We collaborate with hospitals and biomedical companies to translate our technologies to biomedical applications. This includes optical affinity biosensors for the early diagnosis of diseases, active non-thrombogenic coatings for extracorporeal membrane oxygenators, coatings for orthopedic implants, and new concepts for wound dressings that simultaneously protect the wound from bacteria and stimulate its healing.
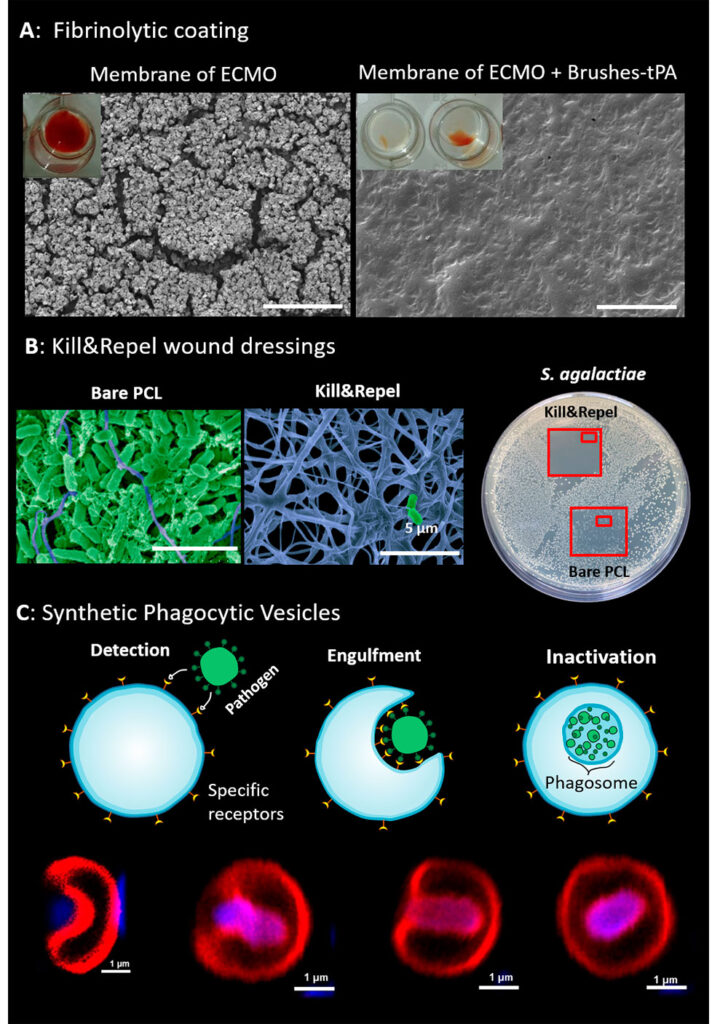
(A) Fibrinolytic adaptive coatings protect the membrane of oxygenators by digesting clots. (B) Kill&Repel coatings for wound dressing prevent the colonization of pathogens and disinfect the wound using powerful endolysins inspired by the antimicrobial activity of bacteriophages. (C) Synthetic phagocytic vesicles engulf and destroy bacteria and viruses.
STAFF
César Rodriguez-Emmenegger
PUBLICATIONS
See full publication list in Google Scholar
Check for more detailed information on the outputs of the Group at IBEC CRIS portal.
Publications list:
COLLABORATIONS
Prof. Virgil Percec (UPENN)
Prof. Andreas Herrmann (DWI)
Prof. Ulrich Schwaneberg (DWI)
Prof. Igor Potemkin (MSU)
PD. Dr. Tamás Haraszti (DWI)
Prof. Petra Schwille (MPI)
PD. Dr Rumiana Dimova (MPI)
Prof. Mike Klein (Temple U.)
Prof. Christopher Barner-Kowollik (QUT)
Prof. Gerhard Gompper (Jülich)
Prof. Gerard Lligadas (URV)
PD. Dr. Dr. Michael Hirtz (KIT)
Prof. Keith E. Cook (CMU)
Prof. Dr. Jakub Dostalek
Dr. Tomas Riedel (IMC)
PD. Dr. Oliver Grottke (UKA)
Prof. Filipe Mergulhão (LEBAPE)
NEWS

The IBEC and West China Hospital hold their third joint conference on precision medicine in Barcelona
Barcelona hosted the third IBEC-WCH Precision Medicine Conference this week, an event that further strengthened the strategic alliance between the Institute for Bioengineering of Catalonia (IBEC) and the West China Hospital of Sichuan University (WCHSU). The Chinese delegation visited Spain from 26 to 29 November, taking part in a busy schedule of scientific and institutional activities and exchanges between the two centres.

Bioengineering for precision medicine at the 18th IBEC Symposium
The 18th annual IBEC Symposium focused on ‘Bioengineering for Precision Medicine’, which is one of IBEC’s key areas of application. The event was attended by nearly 300 people, including local and international researchers. The multidisciplinary environment provided experts from other centres and the IBEC community with the opportunity to present their projects and exchange knowledge.

IBEC and UNIST Host Forum on Next-generation Bioengineering to Strengthen International Collaboration
Today took place the Forum on Next-generation Bioengineering organized by the Institute for Bioengineering of Catalonia (IBEC) and Ulsan National Institute of Science & Technology (UNIST) in South Korea. The event highlighted cutting-edge research in bioengineering and reinforced the collaborative ties between the two institutions.

IBEC and ICMS meet again in Eindhoven for their annual joint symposium
The joint ICMS-IBEC-MPIP symposium took place today, 24 March. The event was jointly organised by the Institute for Bioengineering of Catalonia (IBEC), the Institute for Complex Molecular Systems (ICMS) and the Max Planck Institute for Polymer Research (MPIP). During the day, researchers from the three centres presented their areas of research, with the aim of strengthening the scientific relations between the institutions.

IBEC stands out at BIST Forum with 4 BIST Ignite projects and a BIST Ignite Award
Today, the BIST Forum, an event that brings together the BIST scientific community, focused this year on the joint initiative of the seven CERCA centres to promote precision medicine in healthy ageing. During the event, the new BIST Ignite projects to promote multidisciplinary research were announced, with IBEC involved in four of the five selected projects. In addition, one of the projects in which IBEC is involved won a BIST Ignite Award.

IBEC and SJD Barcelona Children’s Hospital strengthen their collaboration with a day of translational innovation
The Institute for Bioengineering of Catalonia and the Sant Joan de Déu Barcelona Children’s Hospital have held a joint conference to strengthen collaboration in bioengineering and translational medicine. The event, held this morning at the IBEC, highlighted innovative projects, presented a joint PhD programme and encouraged the exchange of ideas between researchers from both institutions.

IBEC scientist César Rodriguez-Emmenegger awarded prestigious ERC Consolidator Grant
IBEC’s Principal Investigator César Rodriguez-Emmenegger has been awarded an ERC Consolidator Grant. This prestigious European funding supports scientists in the consolidation phase of their research teams, allowing them to pursue innovative scientific ideas. The €2 million over five years will allow Rodriguez-Emmenegger and his team to develop Phagocytic Synthetic Cells (PSCs) to fight antibiotic-resistant pathogens.

IBEC and VHIR hold a collaboration day to promote synergies
The 1st Translational Collaboration Day between the Vall d’Hebron Institute of Research (VHIR) and the Institute of Bioengineering of Catalonia (IBEC), held on 21st November, was an opportunity to learn about the projects and research lines of both institutions and to promote interaction between professionals.

IBEC and BST strengthen ties with Translational Collaboration Day
IBEC and the Blood and Tissue Bank of Catalonia (BST) held a day to explore new collaborations in bioengineering and translational medicine. The meeting, held yesterday at IBEC, highlighted innovative projects, presented a joint PhD programme and strengthened the link between biomedical research and clinical applications.
JOBS

Senior Researcher at the Bioinspired Interactive Materials and Protocellular Systems Research Group
Ref: SR-CR-E //Deadline: 16/11/2025

Postdoctoral researcher at the Bioinspired Interactive Materials and Protocellular Systems Research Group
Ref: PD- CR // Deadline: 25/07/2025

Postdoctoral researcher at the Bioinspired Interactive Materials and Protocellular Systems Research Group
Ref: PD-CR // Deadline: 10/03/2025

Postdoctoral researcher at the Bioinspired Interactive Materials and Protocellular Systems Research Group
Ref: PD_CR // Deadline: 10/10/2023

Predoctoral researcher at the Bioinspired Interactive Materials and Protocellular Systems Research Group (PhD-CR)
Reference: PhD-CR // Deadline: 07/07/2023

Predoctoral researcher at the Bioinspired Interactive Materials and Protocellular Systems Research Group
Ref: PhD-CR // Deadline: 19/05/2023


 ibecbarcelona.eu
ibecbarcelona.eu