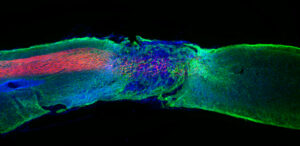
Researchers led by Professor Samuel Stupp of Northwestern University (US) and Distinguished Professor Severo Ochoa at IBEC, and with the participation of Zaida Álvarez Pinto, current researcher of the group “Biomaterials for Regenerative Therapies” at IBEC, have developed a new injectable therapy that uses ‘Dancing molecules’ to reverse paralysis and repair tissue after severe spinal cord injuries.
In a pioneering work done at Northwestern University, with Álvarez Pinto as the first author, and published in the prestigious journal Science, researchers administered a single injection of nanofibers into the spinal cord of paralyzed mice. Only four weeks later, the animals regained the ability to walk.
Once therapy performs its function, materials are biodegraded in 12 weeks in nutrients for cells and then disappear entirely from the body without notable side effects. This is the first study in which researchers control the collective movement of molecules through changes in the chemical structure of nanofibers to increase therapeutic efficiency.
Life expectancy has not improved since 1980
According to the National Spinal Cord Injury Statistical Center, nearly 300,000 people currently live with some kind of spinal cord injury in the US. Less than 3% of those suffering from complete injuries regain basic physical functions and nearly 30% are re-hospitalized at least once, which entails a very high cost in health care. In addition, life expectancy is significantly reduced and has not improved since the 1980s.
“Currently, there are no therapies that trigger the regeneration of spinal cord”, according to Stupp, a regenerative medicine expert and Director of the Simpson Querrey Institute for BioNanotechnology at Northwestern University in Chicago. “I wanted to make a difference on the outcomes of spinal cord injury and to tackle this problem, given the tremendous impact it could have on the lives of patients. Also, new science to address spinal cord injury could have impact on strategies for neurodegenerative diseases and stroke.”
The aim of the study has been to find therapy that can prevent people from being paralysed after a trauma or serious illness in the spinal cord. For decades, this has been a great challenge to scientists because the central nervous system, which includes the brain and spinal cord, has no significant ability to repair after injury or after the start of a degenerative disease.
We are going straight to the FDA to start the process of getting this new therapy approved for use in human patients, who currently have very few treatment options.
Samuel I. Stupp de Northwestern/IBEC
‘Dancing molecules’ hit moving targets
Cells and their receptors are in constant motion, so what researchers have imagined is that if the molecules used in the treatment move faster, they will find these receptors more frequently. On the other hand, slow, “non-sociable” molecules will probably never contact cell receptors.
Injected as a liquid, the therapy immediately gels into a complex network of nanofibers that mimic the extracellular matrix of the spinal cord. By matching the matrix structure, imitating the movement of biological molecules and incorporating signals for receptors, synthetic materials can communicate with cells.
The secret behind this new revolutionary therapeutic treatment is to tune the movement of molecules so that they can properly find and activate constantly moving cellular receptors.
Zaida Álvarez Pinto, First author of the study and currently researcher at IBEC.
“The key innovation in our research, which has never been done before, is controlling the collective movement of more than 100,000 molecules within our nanofibers. By making the molecules move, ‘dance’ or even leap temporarily out of these structures, known as supramolecular polymers, they are able to connect more effectively with receptors, which move constantly” explains Stupp.
Stupp and his team found that adjusting the movement of molecules within the nanofiber network to make them more agile resulted in greater therapeutic efficiency in paralyzed mice. They also confirmed that formulations of their therapy with enhanced molecular motion performed better during in vitro tests with human cells, indicating increased bioactivity and cellular signalling.
An injection, several signals
By sending bioactive signals to trigger cells to repair and regenerate, the breakthrough therapy dramatically improved severely injured spinal cords. Once connected to receptors, moving molecules trigger cascade signals, critical for the repair of the spinal cord.
On the one hand, they induce the regeneration of the axons of the injured spinal cord neurons. Like electric cables, axons send signals between the brain and the rest of the body, and if they suffer cuts or damage, they cause loss of sensitivity in the body or even paralysis.
On the other hand, it helps neurons survive after injury, causes other cellular types to proliferate and promotes the growth of lost blood vessels that feed neurons and critical cells for tissue repair. Therapy also induces the reconstruction of myelin around the axons (the insulating layer important for transmitting electrical signals) and reduces glial scarring, which acts as a physical barrier that prevents the spinal cord from healing.
The signals used in the study imitate the natural proteins needed to induce desired biological responses. However, proteins have extremely short average lives, and their production is very expensive.
“Our synthetic signals are short, modified proteins that — when bonded together by the thousands — will survive for weeks to deliver bioactivity. The end result is a therapy that is less expensive to produce and lasts much longer” explains Zaida Álvarez Pinto, the first author of the work and researcher at IBEC.
Universal Application
While the new therapy could be used to prevent paralysis after further trauma (motor accidents, falls, sports accidents and bullet wounds), as well as diseases, probably the underlying discovery, related to “supramolecular movement” as a key factor in bioactivity, may be applied to other therapies and targets.
“The tissues of the central nervous system that we have successfully regenerated in the injured spinal cord are similar to those of the brain affected by stroke and neurodegenerative diseases, such as ALS, Parkinson’s disease and Alzheimer’s disease”, explains Stupp. “Other than that, our fundamental discovery about controlling the movement of molecular assemblies to improve cell signalling could be universally applied to different biomedical objectives”, concludes.
Reference article:
Zaida Alvarez, Alexandra N. Kolberg-Edelbrock, Ivan R. Sasselli, J.Alberto Ortega, Ruomeng Qiu, Zois Syrgiannis, Peter A. Mirau, Feng Chen, Stacey M. Chin, Steven Weigand, Evangelos Kiskinis, Samuel I. Stupp. Bioactive scaffolds with enhanced supramolecular motion promote recovery from spinal cord injury. Science, 2021, Vol 374 (6569), pp.848-856.
About Samuel I. Stupp:
Samuel Stupp is p rofessor at the Northwestern University in Chicago, USA, and Visiting Severo Ochoa Research DistinguishedProfessor at IBEC. Stupp is Board of Trustees Professor of Materials Science and Engineering, Chemistry, Medicine and Biomedical Engineering at Northwestern, where he is founding director of the Simpson Querrey Institute for BioNanotechnology (SQI) and its affiliated research center, the Center for Regenerative Nanomedicine. Stupp also directs the Center for Bio-Inspired Energy Science, an Energy Frontiers Research Center funded by the U.S., focused on interdisciplinary research. He is also a member of the National Academy of Engineering, the American Academy of Arts and Sciences, the Spanish Royal Academy, and the National Academy of Inventors, and has appointments in the McCormick School of Engineering, Weinberg College of Arts and Sciences and Feinberg School of Medicine. Samuel Stupp has been Director of IBEC’s Scientific Committee (ISC) since 2013.
About Zaida Álvarez Pinto:
She performed her doctoral thesis in biomaterials for repairing brain damage at the UPC/IBEC 2009-2014. During the period 2015-2019, she made a postdoctoral stay at the Simpson Querrey Institute for BioNanotechnology (SQI) at Northwestern University in Chicago. From 2019–2021 she was Research Assistant Professor at the Feinberg School of Medicine at Northwestern University. Recently, as a researcher with a fellowship “Beatriu de Pinós” at the Institute for Bioengineering of Catalonia (IBEC), she obtained a “Ramón y Cajal” grant to continue her research on new therapies for repairing the damaged spinal cord.







