ABOUT
Research in the Biomaterials for Regenerative Therapies group is devoted to the development and knowledge transfer to industry of innovative biomaterials and scaffolds for tissue regeneration.
We design, fabricate and characterize bioactive and biodegradable materials and investigate their interactions with biological entities, both in terms of their fundamental aspects and with specific applications for tissue engineering purposes in mind. The aim is the repair and functional restoration of tissues or organs by means of 3D scaffolds, cells and signals.
Our research is built up in three pillars:
- Creating microenvironments for tissue regeneration. Understanding the interaction of the designed scaffolds with the cells is the key to induce a regenerative environment. We have developed models that explain the activation of angiogenesis and neurogenesis promotion based on the biomaterials properties and the structure of the scaffold, together with the degradation products that promote a bioactive environment. During 2018 we have studied the activation of progenitor cardiac cells, recruitment and differentiation in response to these scaffolds by means of instructive matrices.
- Translating basic research towards new developed products. We are applying Key Enabling Technologies in Biomaterials fabrication to give solutions to companies. A personalized maxillofacial substitute using 3D printing is being developed together with Avinent Implant system, SL. A new dressing that promotes the fast healing of chronic wounds, promoting cell recruitment and regeneration is now under preclinical studies.
- Disease models. We are developing 3D models for tumor research based on cells self-produced extracellular matrices. These microtissues can be modified to culture cancer cells to simulate an ex vivo tumor that can be used for basic cancer research or drug screening. We are also developing novel bioinks for the fabrication of bioprinted platforms as 3D models and bioengineered constructs. The use of microfluidics simulating a vessel, or a cardiac tissue is another type of model that help to make a step forward on cardiac regeneration.
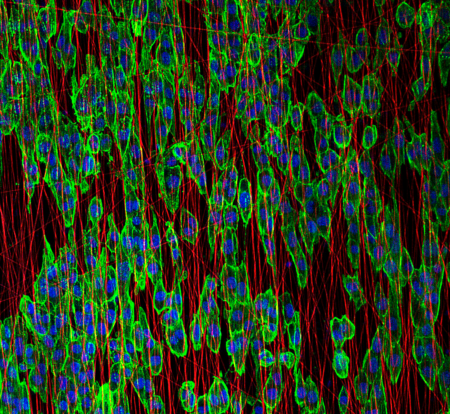
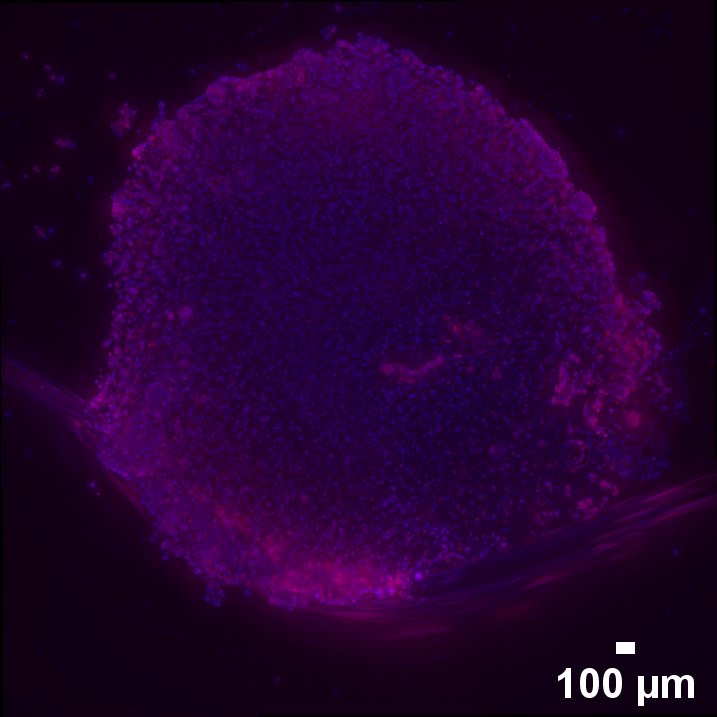
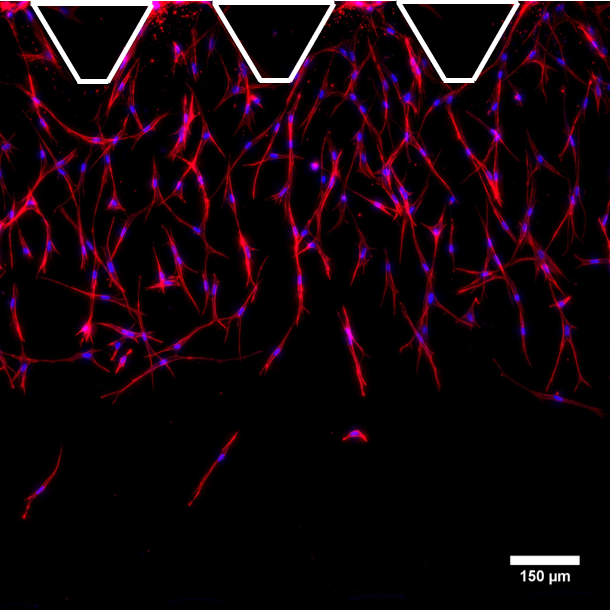
We are members of CIBER-BBN (Centro de Investigación Biomédica en Red – Bioingeniería, Biomateriales y Nanomedicina)
We are participants of the RIS3CAT LLAVOR 3D Community, funded by ACCIO, that aims to accelerate the development and adoption of additive manufacturing and 3D printing* technologies by the industry.*
We are new members of the Red de Terapia Celular (Tercel) to collaborate with the cell therapy groups in tissue and organ regeneration.
| *QuirofAM. Ecosistema d’R+D+i per la implementació i adopció de la Fabricació Additiva / Impressió 3D a la indústria de Salut (2018-2021). El proyecto QuirofAM está enmarcado dentro de la comunidad Llavor 3D, impulsada por la Generalitat de Catalunya para acelerar y desarrollar la adaptación de la fabricación aditiva en el sector industrial y cofinanciada mediante el programa operativo FEDER Catalunya 2014-2020. El objetivo del proyecto QuirofAM es la transformación de la práctica quirúrgica mediante la incorporación de la fabricación aditiva en tres niveles: modelos de ensayo quirúrgico, guías e implantes para reconstrucción e implantes bioactivos para la generación de tejidos. Uno de los beneficios más interesantes que tiene esta tecnología en el sector biomédico es la individualización de tratamientos. |
STAFF
Former Member
Prof. Josep Planell | Director, Universitat Oberta de Catalunya (UOC)
PROJECTS
| NATIONAL GRANTS | FINANCER | PI |
|---|---|---|
| DERMOGLASS · Desenvolupament d’un producte sanitari amb partícules inorgàniques pel tractament de ferides (2021 – 2023) | ACCIÓ, INNOTEC | Elisabeth Engel |
| DERMOGLASS · Fabricació i avaluació d’un prototip d’apòsit per a la cicatrització de ferides (2020-2022) | AGAUR · Ajuts Producte destinats a l’obtenció de prototipus i a la valorització i transferència dels resultats d’investigació generada per equips de recerca de Catalunya. | Elisabeth Engel |
| nAngioDerm · Materiales liberadores de iones para promover la angiogénesis en regeneración dérmica (2019-2022). | MINECO, Acciones de Programación Conjunta Internacional | Elisabeth Engel |
| BASE3D · Agrupació en tecnologies emergents en fabricació aditiva (2019 – 2022) | RIS3CAT · Tecnologies Emergents | Josep Samitier |
| BIOCARDIO · Bioingeniería de constructos basados en biomateriales para la regeneración cardiaca (2019-2021). | MICIU, Retos investigación: Proyectos I+D | Elisabeth Engel |
| TERCEL · Red de terapia celular (2018-2022). | MINECO – ISCIII, Redes temáticas de investigación cooperativa en salud | Elisabeth Engel |
| PRIVATELY FUNDED PROJECTS | FINANCER | PI |
|---|---|---|
| DERMOGLASS · Smart dressing for the treatment of chronic wounds (2019-2022). | Obra Social “la Caixa” · CaixaImpulse | Elisabeth Engel |
| FINISHED PROJECTS | FINANCER | PI |
|---|---|---|
| QuirofAM. Ecosistema d’R+D+i per la implementació i adopció de la Fabricació Additiva / Impressió 3D a la indústria de Salut (2018-2021). | ACCIO, Acreditació de comunitats RIS3CAT i la selecció de projectes col·laboratius de recerca, desenvolupament i innovació | Elisabeth Engel |
| NAngiofrac | Euronanomed | Elisabeth Engel |
| THE GRAIL · Tissue in Host Engineering Guided Regeneration of Arterial Intimal Layer (2012-2016). | HEALTH | Elisabeth Engel |
| DERMOGLASS · Smart dressing for the treatment of chronic wounds (2016-2017). | Obra Social La Caixa, Caixaimpulse, EIT Health | Xavier Puñet |
| Diseño y desarrollo de biomateriales bioactivos para la regeneración de la piel basada en la señalización controlada de liberación de iones (2013-2016). | I+D-Investigación fundamental no orientada | Elisabeth Engel |
| Andamios diseñados para promover una vascularización eficiente para fracturas óseas no consolidadas (2012-2016). | I+D-Investigación fundamental no orientada | Oscar Castaño |
| INSBIOMAT · Biomateriales instructivos para regeneración cardíaca in vivo (2015-2016). | MINECO, Acciones Dinamización “Europa Excelencia” | Elisabeth Engel |
| BIOTENDON · Tendon Tissue Engineering: A Helping Hand for Rotator Cuff Tears. | RECERCAIXA | Elisabeth Engel |
| Nous models de bioimpressió 3D d’os per a ús maxil·lofacial (2017-2019). | MINECO, Retos investigación: Proyectos I+D | Elisabeth Engel |
| MICARE · Creación de microentornos para la regeneración cardíaca in vivo (2017-2019). | MINECO, Acciones Dinamización Europa Investigación | Elisabeth Engel |
| MatriCell · Desarrollo de partículas poliméricas para generar matrices extracelulares in vitro (2016-2019). | MINECO, Retos investigación: Proyectos I+D | Elisabeth Engel |
| State of the Art Research on Expandable Materials (2017-2018). | TUCSE, S.L | Miguel Ángel Mateos |
PUBLICATIONS
EQUIPMENT
- Surface characterization equipment (contact angle, Z potential, nanoindenter)
- Cell culture facilities
- Molecular Biology equipment: protein and DNA electrophoresis
- Thermocycler (PCR)
- Rapid prototyping tool (part of the Production of biomaterials and nanoparticles platform of the CIBER-BBN)
http://www.ciber-bbn.es/programas/plataformas/equipamiento/biomateriales?nodo=nodo2&locale=en - Peptide synthetiser
- Combustion furnace
- Electrospinning device
- Spin-coater
- Vibrational viscosimeter
COLLABORATIONS
- Dr. Ernest Mendoza
Applied Nanomaterials Laboratory, Research Centre in Nanoengineering, Technical University of Catalonia (UPC, BarcelonaTech), Spain - Dr. Izabella Rajzer
Institute of Textile Engineering and Polymer Materials, University of Bielsko-Biala, Poland - Dr. José María Mora
Servei de cirurgia ortopédica i traumatológica, Consorci Hospital de Terrassa, Spain - Dr. Mercè Alsina
Servicio de Dermatología, Hospital Clínic de Barcelona, Spain - Prof. Didier Letourneur
Laboratoire de Bioingénierie Cardiovasculaire, INSERM, University Denis Diderot-Paris 7, Paris, France - Prof. Dirk Grijpma
Department of Biomaterials Science and Technology, University of Twente, Twente, the Netherlands - Prof. Francesco Serino
Department of Vascular Surgery, Istituto Dermatologico dell’Immacolata (IDI), Rome, Italy - Dr. Jerónimo Blanco
Institut de Ciències Cardiovasculars de Catalunya and CSIC, Barcelona, Spain - Dr. Joelle Amedee
INSERM, University of Bordeaux Segolen, Bordeaux, France - Dr. José Becerra Ratia
Dept. Biología Celular, Genética y Fisiología, Universidad de Málaga, Spain - Dr. Margarita Calonge
Institute of Ophthalmobiology (IOBA), Universidad de Valladolid, Spain - Dr. José Carlos Rodríguez-Cabello
Dept. de Física de la Materia Condensada, Universidad de Valladolid, Spain - Prof. Kevin Healy
Biomaterials & Tissue Engineering Laboratory, University of California at Berkeley, USA - Prof. Jaume Veciana
NANOMOL, Institut de Ciència de Materials de Barcelona (ICMAB-CSIC), Spain - Dr. Diego Gutiérrez de la Iglesia (MD)
Pediatric orthopaedic surgery, San Juan de Dios Hospital, Spain - Prof. Wouter J.A. Dhert & Dr. Jos Malda
Department of Orthopaedics, University Medical Center Utrecht, The Netherlands - Prof. Andrés J. García, F.B.S.E.
Institute for Bioengineering and Bioscience, Georgia Institute of Technology, Atlanta, GA - Dr. Luigi Ambrosio
Institute of Polymers, Composites & Biomaterials, National Research Council, Naples, Italy - Prof. Carlos Semino
Grupo de Insuficiencia Cardíaca y Regeneración Cardíaca (ICREC), IQS School of Engineering, Universitat Ramon Llull - Dr. Antonio Sinovas
Vall d’Hebron Institute of Research - Dr. Dimitrios Zeugolis
Biomedical Engineering (Biomaterials) at NUI Galway - Dr. Aitor Aguirre
Department of Biomedical Engineering, MSU, Michigan, US - Prof. Joao Mano
CICECO, Aveiro University, Portugal - Prof. Gino Ambrosio
Università degli Studi di Napoli Federico II: Napoli, Campania, Italy - Prof. Timothy Thomson
IBMB-CSIC, Barcelona - Dr. Elena Rebollo
IBMB-CSIC, Barcelona - Dr. Jordi Camps
Gastrointestinal and Pancreatic Oncology Group, Institut D’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) - Dr. Olivier Stephan
Univ-grenoble-alpes, France - Prof. Humberto Palza
Departamento de Ingeniería Química, Biotecnología y Materiales, Universidad de Chile - Prof. Borros
IQS, Barcelona - Dr. Judith Guasch
ICMAB, Barcelona - Dr. Elena Xuriguera
DIOPMA, University of Barcelona - Dr. Carlos Aleman
EEBE, UPC - Dr. Felip Fenollosa
Centre CIM, UPC - Prof. Aurora Hernandez-Machado
Estructura i Constituents de la Materia, University of Barcelona - Avinent Implant System, SL
Sant Pedor, Spain - Laboratorios ERN
Barcelona, Spain - Microlight3D
France - Vecmedical SL
Barcelona - Dr. Roberto Vélez
Orthopedic oncology and Traumatology department, Vall d’Hebron Hospital - Dr. Joan-Pere Barret
Head of the Plastic Surgery and Burns Service at Hospital Vall d’Hebron - Dr. Antoni Compte Verdeguer
Orthopedic and Traumatology pediatric surgeon, Hospital Sant Joan de Déu, Barcelona, Spain - Dr. Jose María Moraleda
Hospital Virgen de la Arrixaca, Murcia
NEWS
Bioaction Project: Turning Infections from Health Risk to Opportunity
Bioaction is an EU-funded project, in which IBEC is involved, that aims to revolutionize the approach to implant-associated infections. By harnessing hydrogels and smart nanomaterials, Bioaction will transform bacterial biofilms into bio-factories for tissue regeneration, offering a novel alternative to traditional antibiotic therapies. The project is driven by a multidisciplinary team of experts and strives to improve patient outcomes while addressing the global challenge of antimicrobial resistance.
Elisabeth Engel and Gabriel Gomila receive the ICREA Acadèmia grant
IBEC researchers Elisabeth Engel and Gabriel Gomila have been awarded the “ICREA Acadèmia” distinction by the Catalan Institution for Research and Advanced Studies (ICREA). Engel and Gomila, who lead their … Read more
The President of Catalonia visits IBEC
During the visit, the President of the Government of Catalonia, Pere Aragonès i Garcia, and the Minister of Health, Manel Balcells i Díaz, were able to learn first-hand about several … Read more
3D bioprinting on RTVE
Lead researcher Eli Engel and IBEC researcher Bárbara Blanco are featured on RTVE for their recent research against breast cancer. Using 3D bioprinting they have created tumors from pig tissue. … Read more
3D bioprinting and cancer in the media
IBEC researchers led by Elisabeth Engel are in the media for their new tool against breast cancer: 3D bioprinting of tumors using pig tissue. The study, led by group leader … Read more
PostDoc Researcher at the Biomaterials for Regenerative Therapies Research Group
Ref: PostDoc / Deadline: 31st July 2022
A new tool against breast cancer: 3D bioprinted tumors using pig tissue
Researchers at IBEC manage to recreate the complex composition of human breast tumors in the laboratory using tissues from female pig breasts. This new model will allow, among other applications, the production of artificial tumors to study the development of cancer and metastasis, and immunosuppression processes, as well as being an excellent platform for testing drugs against the disease.
Zaida Álvarez wins the Rafael Hervada prize
At the press conference held this morning at the San Rafael Hospital, the Rafael Hervada prize was announced. Zaida Álvarez, current researcher at IBEC, has been awarded for her pioneering research on regeneration of spinal cord injuries using nanotechnology at the Northwestern University, Chicago, USA.
Biomaterials made at IBEC reach the Bulgarian press
The research carried at the “Biomaterials for regenerative therapies” group at IBEC, has reached the international press. In this case, the magazine Cosmos from Bulgary has published an article explaining their research in biomaterials and the use of nanoparticles.
nAngioDerm participated in world congress TERMIS
Professor Elisabeth Engel from IBEC, coordinator of nAngioDerm European project, presented the advances of the project at the 6th world congress of the Tissue Engineering and Regenerative Medicine International Society (TERMIS2021). Professor Engel talked about cells-materials interactions to promote vascularization in one of the most important scientific conferences in the field of tissue engineering and regenerative medicine.
JOBS
Postdoctoral position at the Research Group Biomaterials for regenerative therapies
Ref: PD-EE //Deadline: 18/04/2024
Senior researcher at the Biomaterials for Regenerative Therapies Research Group (SR_EE)
Ref: SR-EE/Deadline: 28/11/2023
Research Assistant at the Biomaterials for Regenerative Therapies Research Group
Ref: EE_RA/Deadline: 21/11/2023
Postdoctoral Researcher at the Biomaterials for Regenerative Therapies Group (PD-EE)
Ref: PD-EE // Deadline: 19/05/2023
Research Assistant at the Biomaterials for Regenerative Therapies Research Group (RA-EE)
Ref: RA-EE // Deadline: 19/05/2023
Research Assistant at the Biomaterials for Regenerative Therapies Research Group
Research Assistant / Deadline October 19th 2022
PostDoc Researcher at the Biomaterials for Regenerative Therapies Research Group
Ref: PostDoc / Deadline: 31st July 2022
PostDoc Researcher at the Biomaterials for Regenerative Therapies Research Group
Application Deadline: 23/11/2021Ref: PD-EE The Biomaterials for Regenerative Therapies group at the Institute for Bioengineering of Catalonia (IBEC) is looking for a Postdoc position to work on the development of new platforms for skin regeneration. The contract will be within the framework of the European Consortium nAngioderm, an EuroNanoMed 3 project funded by the Spanish Ministry of Science and Innovation, whose objective is to develop devices based on nanotechnology and drug delivery tools able to address the healing of acute and chronic wounds.


 ibecbarcelona.eu
ibecbarcelona.eu