About
The Bacterial infections: antimicrobial therapies group is a senior group.
Infectious diseases constitute a tenacious and major public health problem all over the world. The emergence and increasing prevalence of bacterial strains that are resistant to available antibiotics demand the discovery of new therapeutic approaches.
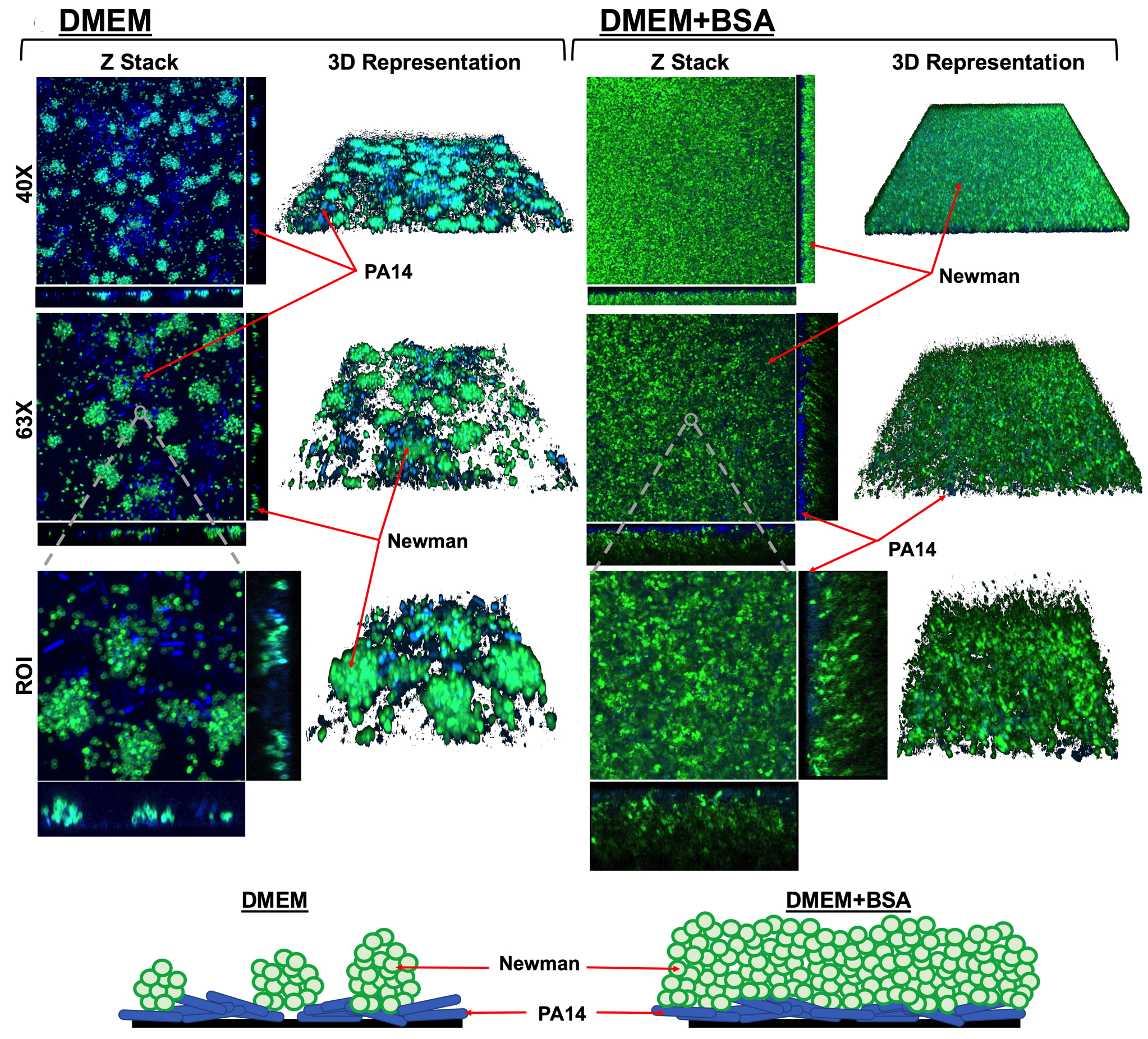
Biofilms are bacterial communities that grow embedded within a protective matrix produced by themselves.
Chronic infections caused by bacteria growing in biofilms, are enormously complicated to treat. It increases their fitness and survival, thus complicating treatment and diagnosis because they persist despite the action of antibiotic therapies and adaptive immune responses.
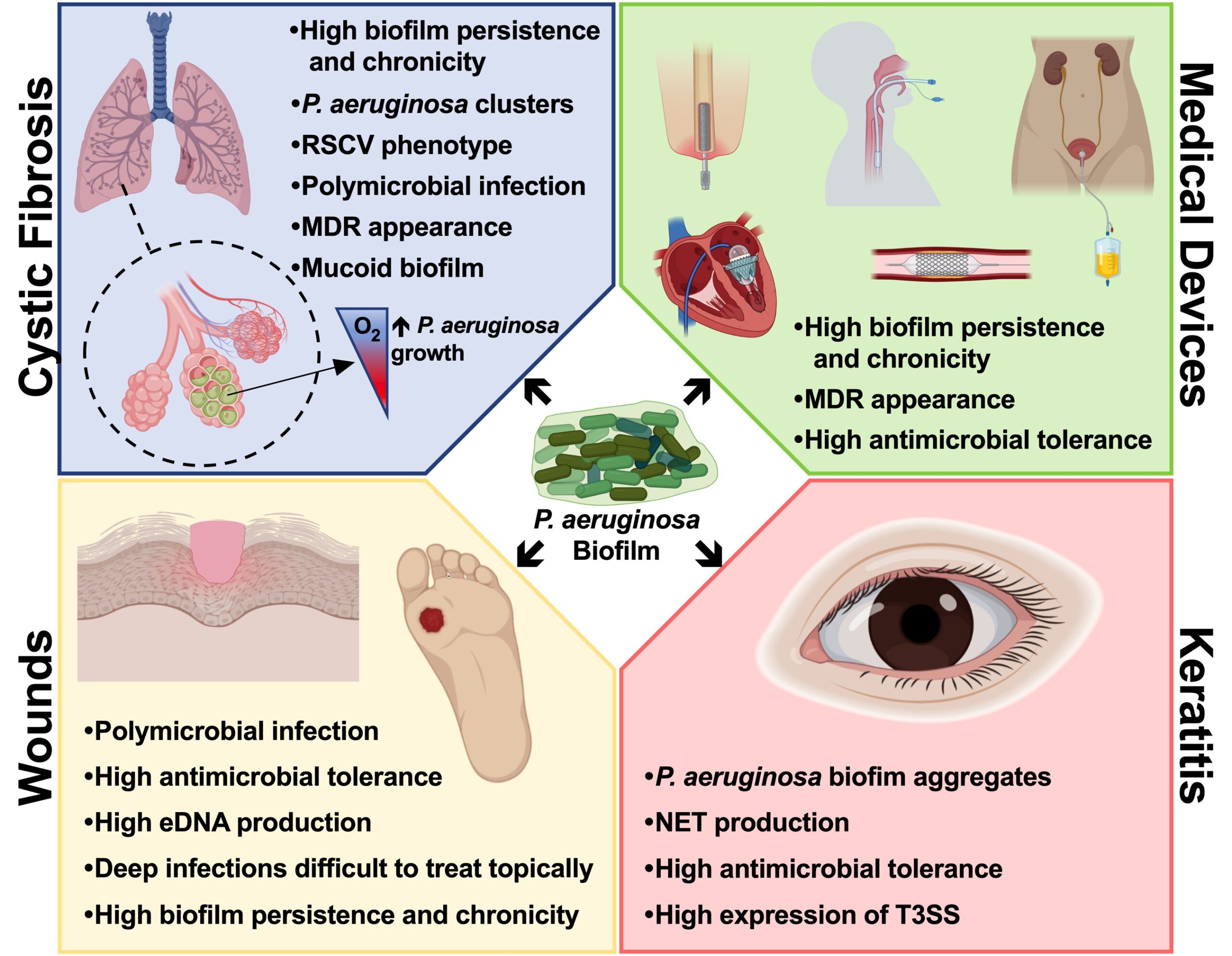
Over 60% of all human infections are characterized by the formation of a biofilm, which is involved in a wide variety of pathological conditions by either growing over human tissues (Cystic Fibrosis, Chronic Obstructive Pulmonary Disease, chronic wound, etc.) or by developing on the surfaces of medical devices (e.g. endotracheal tubes, intravenous and urinary catheters, etc.).
Our lab aims to investigate new antimicrobial therapies and strategies to combat bacterial infections with different objectives:
- The use of nanomedicine techniques for the development of novel and specific nanoparticles to deliver existing antibiotics or new identify antimicrobial drugs, significantly when the bacteria are growing in biofilm, close to the physiological conditions of the disease and where the current chemotherapy fails;
- The identification and screening of new molecules for the highly selective inhibition of new antibacterial targets (e.g. ribonucleotide reductases);
- The use of nanomedicine techniques for the development of novel and specific nanoparticles to deliver existing antibiotics or new identify antimicrobial drugs, significantly when the bacteria are growing in biofilm, close to the physiological conditions of the disease and where the current chemotherapy fails;
- To study new methodologies to treat chronic bacterial infections in patients suffering cystic fibrosis;
- To develop a new family of antibacterial vaccines;
- The development of new strategies for bacterial coculture systems;
- To study and develop models for wound healing infections and the search of novel treatments;
- The use of lab-on-a-chip technology to deeply elucidate mechanisms to combat bacterial forming biofilm as well as new approaches to identify multiresistant bacteria to different antibiotics.
- To establish the molecular basis for the regulation of genes involved in DNA synthesis (ribonucleotide reductase genes), their importance in virulence and biofilm formation;
We believe these projects will be beneficial to society since we explore the use of different bioengineering approaches to elucidate ways to diagnose and eradicate multi-drug resistant bacteria.
Related links:
Staff
Former Members
Maria del Mar Cendra | PhD Student
Projects
| NATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| InfectTreat · Understanding DNA metabolism and new insights in polymicrobial biofilms: development of more efficient therapies to tackle bacterial infections (2022 – 2025) | MICIU. Generación Conocimiento proyectos I+D | Eduard Torrents |
| DISnanoAMR · Desarrollo de nuevas estrategias para hacer frente a la resistencia antibiótica (2022 – 2025) | MICIU. Poryectos de I+D+i en líneas estratégicas | Eduard Torrents |
| IVD-Biofilm · Desarrollo de un nuevo dispositivo para el diagnóstico personalizado en infecciones relacionadas con biopelículas (2022 – 2024) | MICIU. Proyectos Pruebas de Concepto | Eduard Torrents |
| Acuerdo de colaboración ente el IBEC y la Asociación Catalana de Fibrosis Quística (2019 – 2024) | Asociación Catalana de Fibrosis Quística | Eduard Torrents |
| Las biopelículas polimicrobianas para el desarrollo de terapias más eficientes contra las infecciones bacterianas” (2021-2022) | Diputació de Barcelona | Eduard Torrents |
| combatRNR · Comprender la síntesis del ADN en patógenos bacterianos: nuevas estrategias para el tratamiento de enfermedades infecciosas (2019 – 2022) | MICIU. Retos investigación: Proyectos I+D | Eduard Torrents |
| BIOVAC · Artificial bacteria: a novel generation of bioinspired vaccines (2020 – 2023) | BIST. BIST Ignite Program | Eduard Torrents |
| Las biopelículas polimicrobianas para el desarrollo de terapias más eficientes contra las infecciones bacterianas” (2021-2022) | Diputació de Barcelona | Eduard Torrents |
| FINISHED PROJECTS | FINANCER | PI |
|---|---|---|
| Terapias alternativas para el tratamiento de las infecciones bacterianas crónicas en pacientes con fibrosis quística a (2019-2021) | Asociación Catalana de Fibrosis Quística | Eduard Torrents |
| Noves estratègies antimicrobianes per combatre la fibrosi quística (2016-2020) | Obra Social La Caixa | Eduard Torrents |
| BiofilmChip CaixaImpulse BiofilmChip (2018 – 2020) | Obra Social La Caixa | Eduard Torrents |
| Desarrollo de una nueva familia de compuestos antimicrobianos | Asociación Catalana de Fibrosis Quística | Eduard Torrents |
| Identificación y administración de nuevas moléculas antimicrobianas contra Pseudomonas aeruginosa creciendo en biofilm | Asociación Española Fibrosis Quística, Becas de Investigación “Pablo Motos” | Eduard Torrents |
| Novel strategies to combat bacterial chronic infections by the development of microfluidics platforms to analyse and treat bacterial growing in biofilms (2016) | Obra Social La Caixa | Eduard Torrents |
| Redes reguladoras de la expresión génica de las distintas ribonucleotidil reductasas en bacterias | MINECO, I+D-Investigación fundamental no orientada | Eduard Torrents |
| BACTSHOT Novel antimicrobial therapy (2016-2017) | EIT Health Head Start – Proof of Concept | Eduard Torrents |
| inhibitRNR Las ribonucleotido reductasas como una nueva diana terapéutica frente a patógenos bacterianos (2016-2018) | MINECO, Retos investigación: Proyectos I+D | Eduard Torrents |
| Ribonucleotide reductasas: una nueva diana terapéutica contra organismos patógenos en enfermos de fibrosis quística (2010-2017) | Asociación Española Fibrosis Quística, Becas de Investigación “Pablo Motos” | Eduard Torrents |
| RNRbiotic New strategy to combat bacterial infections (2015-2017) | Obra Social La Caixa, Caixaimpulse | Eduard Torrents |
Publications
(See full publication list in ORCID)
[br]
Equipment
- Zeiss LSM 800 Confocal Laser Scanning Microscope
- Nikon Inverted Fluorescent microscope ECLIPSE Ti-S/L100
- Cell culture facilities for microbial infections
- Characterization of nanoparticles/biomaterial antibacterial activity
- Drosophila melanogaster and Galleria mellonella as a model host for bacterial infections
- Continuous flow system model for bacterial biofilm development
- Single Channel Fiber-Optic Oxygen Meter with microsensor
- Molecular biology, biochemistry and protein purification facilities
- Bacterial expression systems for heterologous protein production
Collaborations
- Prof. Daniel Ruiz
Català de Nanociència i Nanotecnologia (ICN2), Barcelona, Spain - Prof. Bianca Sclavi
NRS Biologie Computationnelle et Quantitative. Sobornne Université. Paris. France - Dr. Esther Julián
Dept. de Genètica i de Microbiologia, Universitat Autònoma de Barcelona, Spain - Prof. Josep Samitier
IBEC - Prof. Santiago Vazquez
Laboratori de química farmacèutica, Pharmacy Faculty, Barcelona University - Prof. Gabriel Gomila
IBEC - Dr. Maarten Fauvart
IMEC, Leuven, Belgium
News

New Faster Future campaign tackles bacterial resistance
IBEC dedicates the 4th edition of its fundraising program, Faster Future, to develop novel strategies to treat infections and diseases caused by antibiotic-resistant bacteria. Specifically, the research seeks to fight … Read more

New strategy against chronic wound infections
Chronic wounds are a challenge for microbiologists due to biofilms, complex structures where bacteria protect themselves from antibiotics, preventing infections from being treated effectively. A research team led by the … Read more
Researchers developed three molecules that improve the efficacy and reduce the toxicity of existing antibiotics
IBEC researchers have published a study in the journal Nature in which they have designed and developed a three-molecule complex that increases the efficacy and stability, while reducing the toxicity, … Read more

IBEC researchers discover how to improve the existing antibiotics and develop new antimicrobial treatments
In a new study carried out by researchers from the Institute for Bioengineering of Catalonia (IBEC), improved so that they are more effective in much smaller doses, which opens the … Read more
3D mini-intestines to study bacterial infections
Researchers at IBEC have developed a 3D model of the human intestine that simulates the characteristics of the intestinal mucosa and its reliefs. These mini-intestines will make it possible to … Read more

Breaking down bacteria’s protective armor to overcome antibiotic resistance
A new triple-acting antibiotic agent has managed to break through the biofilm extracellular matrix – a protective structure built by bacteria – and eliminate more than 50% of the pathogens … Read more
Researchers succeed in breaking the shield of bacteria that makes them resistant to antibiotics
The group led by Eduard Torrents has succeeded in developing a triple-action antibiotic that breaks a protective structure produced by the bacteria themselves, the bacterial biofilm. Using this new antibiotic, … Read more

Nanomedicine seeks solutions against rare diseases
Despite its importance in the fight against pandemics such as COVID-19, a lesser-known face of nanomedicine is its potential to contribute to solutions to so-called rare or minority diseases. Coinciding with February 28th, the world day for rare diseases, experts invited by the Nanomed Spain platform and the Sant Joan de Déu Research Institute (IRSJD) present the latest advances in nanomedicine against three of these disorders: muscular dystrophy, cystic fibrosis and Fabry disease.

BiofilmChip protagonist in the media
Researchers from the Institute for Bioengineering of Catalonia (IBEC), led by Eduard Torrents, leader of the group “bacterial infections and antimicrobial therapies” and professor at the University of Barcelona (UB), in collaboration with Josep Samitier, principal investigator of the group “Nanobioingineering” of IBEC, and Maite Martin, of the Vall d’Hebron Barcelona Hospital appear in the media for the new device, called BiofilmChip, which allows a custom and precise diagnosis of chronic infections.

A chip against chronic bacterial infections
IBEC researchers develop a device that allows to grow biofilms and analyze their susceptibility to different antibiotics in a simple way and using patient samples. BiofilmChip, a low-cost, easy-to-use diagnostic device, opens the way to finding effective and custom treatments against chronic infections produced by biofilms.


 ibecbarcelona.eu
ibecbarcelona.eu