Researchers from the Institute of Bioengineering of Catalonia (IBEC) and the University of Barcelona (UB) uncovered a similarity between liquid droplets and cell groups, revealing that surface tension helps cells to migrate towards stiffer environments. This new insight could help us to understand how cancer cells disseminate across tissues with different rigidity in our body.
Scientists have long investigated the theory that the behavior of cells, the building blocks of our body, can be explained through the physics of fluid droplets. A recent study by scientists from the Institute of Bioengineering of Catalonia and the University of Barcelona has contributed new information to this area of research. The results, published recently in the journal Nature Physics, demonstrate that physics describing how a droplet actively moves and wets a surface can also explain how clusters of cancer cells migrate from soft to stiffer environments. This discovery could help us understand how cancer cells spread throughout the body.
Cells move through the body for different purposes: to create new organs during embryonic development, to chase pathogens, and also to spread tumors during metastasis. It is known that, to orient themselves, cells can detect mechanical signals, such as the stiffness of their environment, and also that certain cells migrate from softer to stiffer environments, in a process known as durotaxis. Durotaxis is most efficient when performed collectively, that is, when cells move in groups, but the physics behind collective durotaxis is still poorly understood.
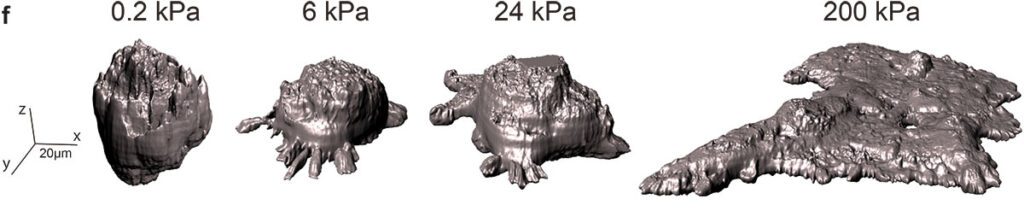
A research team co-led by Xavier Trepat, ICREA research professor at IBEC, in collaboration with researchers from the University of Barcelona (UB), the Max Planck Institute and CIBER-BBN, found that, as the group of cancer cells migrated to stiffer surfaces, the cells first accelerated, but then slowed down, concluding that there is optimal rigidity at which cells migrate faster. “We also observed that the cell groups adopted different shapes depending on the rigidity of the surface,” explains Macià Pallarès, co-author of the study during his PhD at IBEC.
Most theories to understand this behavior do not take into account the three-dimensional shape of the group of cells. To address this limitation, the team developed a new 3D model. Given the similarities in shape and behavior between cell groups and living droplets, the team developed a theory of cell clusters as living droplets that can propagate and move over surfaces. “Using the physics of surface tension, which is the force that makes water droplets spherical, we were able to explain the shapes and movement of groups of cells,” says Irina Pi Jaumà, co-author of the study and researcher in the Department of Condensed Matter Physics of the University of Barcelona, who participated in the development of the theory.
This finding shows that the physics of wetting, when generalized to living liquids, provides a way to understand cell migration without needing to consider the complexities of cell-cell communication.
“What we propose is that this process, durotaxis, which was defined in the field of cell biology, can be explained quite precisely with the physics of wetting,” says Xavier Trepat, ICREA researcher at IBEC.
The findings of this study have the potential to lead to new treatments and therapies for cancer patients, by providing a better understanding of how cancer cells migrate and spread.
Referenced article:
Esteve Pallarès, M., Pi-Jaumà, I., Corina Fortunato, I., Grazu, V., Gómez-González, M., Roca-Cusachs, P., de la Fuente, J.M., Alert, R., Sunyer, R., Casademunt, J., & Trepat, X. Stiffness-dependent active wetting enables optimal collective cell durotaxis. Nature Physics (2022). DOI: 10.1038/s41567-022-01835-1





