ABOUT
Tissues in our body can be extremely soft such as breast or brain, or very stiff such as bone. Cells in our body constantly interact mechanically with such tissues, exerting, transmitting, withstanding, and detecting forces. This mechanical interaction with the environment regulates how cells proliferate, differentiate, and move, and regulates development, tumorigenesis or wound healing.
Our research aims at unraveling – and re-engineering – the molecular mechanisms by which cells detect and respond to mechanical stimuli like forces or tissue rigidity, triggering downstream cell responses.
Just like biochemical stimuli initiate signaling cascades, mechanical forces affect the links and conformation of a network of molecules connecting cells to the extracellular matrix. This molecular and cellular response to force constitutes the phenomenon of mechanotransduction.
To study mechanotransduction, we combine biophysical techniques like magnetic and optical tweezers, Atomic Force Microscopy, traction microscopy, and microfabricated force sensors with molecular biology, advanced optical microscopy, and theoretical modelling.
Sensing the environment: Using this multi-disciplinary approach, we have unveiled a molecular mechanism that cells employ to detect and respond to the rigidity of their environment, which could be crucial in breast tissue and breast cancer (Elosegui-Artola et al., 2016 Nat. Cell Biol., and Elosegui-Artola et al. 2014, Nature Mater.). This mechanism is mediated by what is known as a “molecular clutch”: in a surprising analogy with a car engine, cells can be understood as a molecular network that can engage and disengage from its environment, just as the clutch of a car. This affects force transmission from the environment to cells, and also within different cell components. We are also expanding on the idea of the molecular clutch, to explore how cell molecular engines sense not only mechanical rigidity, but other important parameters from their environment: for instance, the composition and distribution of ligands in the extracellular matrix, or other cells. In this regard, we uncovered that this concept can explain how cells sense the spatial distribution of ligands in the extracellular matrix (Oria et al., Nature 2017). We have also demonstrated that cell-cell force transmission, mediated by a molecular clutch, is essential for cells to sense gradients in stiffness (Sunyer et al., Science 2016, in collaboration with the group of Xavier Trepat).
Nuclear mechanotransduction: Forces applied to cells are transmitted all the way to the cell nucleus, where they affect its function. We are studying how this force transmission affects the dynamics of transcriptional regulators, such as YAP (Elosegui-Artola et al., 2017, Cell), and how this affects cell function.
The membrane as a mechanosensor: Due to its mechanical properties, the plasma membrane itself can respond to forces and act as a mechanosensor. Recently, we have shown that cell membranes can use purely physical principles to adapt their shape in response to mechanical forces (Kosmalska et al., 2015, Nat. Commun.). We are currently studying how cells harness this physical membrane behavior to respond to signals from their environment.
Ultimately, when we determine the molecular mechanisms that communicate cells with their environment, we will understand how forces determine development when things go right, and tumor formation when they go wrong.
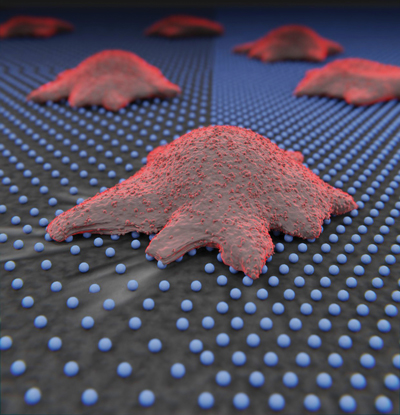
Video: How tissue stiffness activates cancer
STAFF
The following is a list of the current staff members of the research group:
PROJECTS
| NATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| MECNUC · Estudio del control mecánico de la localización nuclear de proteínas (2020-2023) | MINECO Retos investigación: Proyectos I+D | Pere Roca-Cusachs |
| BLOCMEC Development of small molecules to block mechanotransduction for pancreatic cancer therapy (2021-2023) | MICIU, Proyectos Pruebas de Concepto | Pere Roca-Cusachs |
| INTROPY INhibiting mechanoTRansduction for Oncology theraPY (2021-2023) | ACCIO, Tecniospring Industry | Mamatha Nijaguna |
| INTERNATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| MECHANOCONTROL · Mechanical control of biological function (2017-2021) | European Commission, FET Proactive | Pere Roca-Cusachs |
| TALVIN · Inhibiting mechanotransduction for the treatment of pancreatic cancer (2018-2021) | European Commission, FET Innovation Launchpad | Pere Roca-Cusachs |
| MECHANOSITY Mechanical regulation of cellular behaviour in 3D viscoelastic materials (2019-2022) | European Commission, MARIE CURIE | Alberto Elosegui |
| PRIVATELY-FUNDED PROJECTS | FINANCER | PI |
|---|---|---|
| Mech4Cancer · Enabling technologies to map nuclear mechanosensing: from organoids to tumors (2020-2023) | Obra Social La Caixa Health Research Call | Pere Roca-Cusachs |
| Understanding YAP-mediated mechanotransduction in pancreatic cancer (2020-2023) | Fundació La Marató de TV3 | Pere Roca-Cusachs |
| Understanding and measuring mechanical tumor properties to improve cancer diagnosis, treatment, and survival: Application to liquid biòpsies (2017-2022) | Obra Social La Caixa | Pere Roca-Cusachs |
| FINISHED PROJECTS | FINANCER | PI |
|---|---|---|
| Desarrollo de una terapia innovadora para el tratamiento de los tumores sólidos mediante la inhibición de la mecanotransducción (2018-2020) | MINECO, Subprograma Retos-Colaboración | Pere Roca-Cusachs |
| Understanding and measuring mechanical tumor properties to improve cancer diagnosis, treatment, and survival: Application to liquid biopsies (2017-2020) | Obra Social La Caixa | Pere Roca-Cusachs |
| IMREG El sistema acoplado entre integrinas y proteínas adaptadoras como regulador mecánico del comportamiento celular (2016-2020) | MINECO, Proyectos I+D Excelencia | Pere Roca-Cusachs |
| MECHANOMEMBRANE Redes mecanoquímicas en la membrana plasmática (2017-2018) | MINECO, Subprograma Estatal de Generación de Conocimiento “EUROPA EXCELENCIA” | Pere Roca-Cusachs |
| Stromal stiffness in tumor progression (2014-2017) | Fundació La Marató de TV3 | Pere Roca-Cusachs |
| MECBIO Red de Excelencia en Mecanobiología (2014-2016) | MINECO, Subprograma Estatal de Generación de Conocimiento “REDES DE EXCELENCIA” | Pere Roca-Cusachs |
| Inhibiting mechanostransduction as a novel therapy in the treatment of solid tumors (2017-2018) | Obra Social La Caixa | Pere Roca-Cusachs |
PUBLICATIONS
Click here for a list of publications by Pere Roca-Cusachs with IBEC affiliation.
Click here for a full list of publications including those affiliated to other organisations.
EQUIPMENT
- Confocal Microcopy
- Traction Microscopy
- Live cell fluorescence microscopy
- Cell stretching
- Cell culture
- Magnetic Tweezers
- Atomic Force Microscopy
- Surface Micro/Nano-patterning
- Optical tweezers
COLLABORATIONS
- Dr. Nils Gauthier
Mechanobiology Institute, Singapore - Prof. Miguel Ángel del Pozo
Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid - Prof. Marino Arroyo
UPC, Barcelona - Prof. Ada Cavalcanti
University of Heidelberg, Germany - Satyajit Mayor
National Centre for Biological Sciences, Bangalore, India - Sergi Garcia-Manyes
King’s College, London, UK - Louise Jones
Barts Cancer Institute, London, UK - Aránzazu del Campo
INM Saarbrücken, Germany - Patrick Derksen
UMC Utrecht, the Netherlands - Johanna Ivaska
University of Turku, Finland - Jacco van Rheenen
Netherlands Cancer Institute, Netherlands - Isaac Almendros and Ramon Farré
UB, Barcelona - Marc Martí-Renom
CNAG, Barcelona - Marc Güell
UPF, Barcelona - Francisco Real
CNIO, Madrid - Jonas Ries
Max Perutz labs, Vienna
Clinical collaborations
- University Medical Centre Utrecht
- Vall d’hebron Institute of Oncology
NEWS

Success at the conclusion of the 6th edition of the course “Mad for Bioengineering”
For the sixth consecutive year, IBEC has successfully hosted its ‘Mad for Bioengineering’ course with the support of the Catalunya La Pedrera Foundation. Geared towards 1st-year high school students interested in STEM careers, the program provides a unique immersion in the field of bioengineering, addressing health issues from a multidisciplinary perspective. The closing ceremony, attended by students and their families, featured presentations of final projects and the awarding of diplomas.
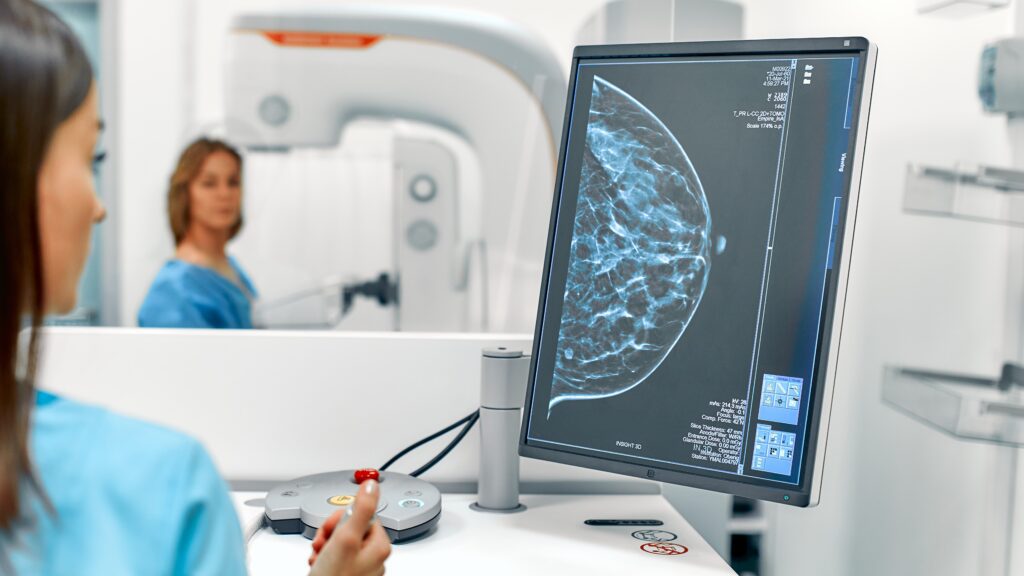
Preventing the tissue’s response to stiffness may be key to slowing the progression of breast tumors
A study led by the Institute of Bioengineering of Catalonia demonstrates that laminin, a protein present in breast tissues, prevents the effects of stiffening, protecting cells against tumor growth. While the mechanism has been demonstrated in vitro, persuasive indications suggest its potential applicability in vivo, as observed in patient samples.

Daniel Navajas: 30 years dedicated to mechanobiology
Last May 5, on the occasion of Professor Daniel Navajas’ retirement, the IBEC held the symposium Before Mechanobiology had a name. The event paid tribute to the IBEC researcher’s exciting … Read more
Researcher Pere Roca-Cusachs has been awarded the European ERC Advanced Grant
IBEC researcher Pere Roca-Cusachs has been awarded an ERC Advanced Grant, one of the EU’s most prestigious and competitive grants. This type of funding allows established researchers to carry out … Read more

The researcher Pere Roca-Cusachs awarded a prestigious European ERC Advanced Grant
The IBEC researcher has been awarded an ERC Advanced Grant, one of the most prestigious and competitive funding in the EU that allows consolidated researchers to carry out ambitious research … Read more

Pere Roca against cancer progression in the Big Vang
Pere Roca-Cusachs, principal investigator at IBEC, appears in the Big Vang section of La Vanguardia for his research on cellular mechanotransduction.

Mechanosensing in the media
IBEC researchers led by Pere Roca-Cusachs and Xavier Trepat appears in the media for a study that opens doors for new research into cancer therapies and diagnostics.
Mechanosensing: harnessing nuclear mechanics to understand health and disease
A study led by IBEC researchers, and published in Nature Cell Biology, shows that applying mechanical force to the cell nucleus affects the transport of proteins across the nuclear membrane. In doing so, this controls cellular processes and could play a key role in various diseases, such as cancer. This entails a novel approach to understanding aspects of cancer invasion and metastasis, opening the door to potential new techniques for diagnosis and therapy.
Researchers discover how cellular membranes change curvature depending on BAR proteins
A team of researchers at IBEC and UPC, led by Pere Roca-Cusachs and Marino Arroyo, study how BAR proteins, a family of molecules that bind curved cellular membranes, reshape these membranes. Scientists report in the journal Nature Communications, through both experiments and modelling, the dynamics of these membrane reshaping processes that occur both in normal cells or disease scenarios.

“Cellular push and pull”, protagonist also in the media
Researchers led by Pere Roca-Cusachs appear in different media for their study published in the prestigious journal “Nature Communications” that discovers how force dynamics affect cells, and living tissues.
JOBS
Postdoc position at the Cellular and Molecular Mechanobiology Research Group
Ref: PD_PR //Deadline: 20/09/2023
Postdoctoral at the Cellular and Molecular Mechanobiology Research Group Unit
Ref: PR_PR// Deadline: 23/06/2023
Laboratory Technician at the Cellular and Molecular Mechanobiology Research Group (LT_PR)
Ref: LT_PR // Deadline: 30/06/2023
Research Assistant at the Cellular and Molecular Mechanobiology Research Group
Research Assistant/ Deadline: November 15th, 2022.
Research Assistant at the Cellular and Molecular Mechanobiology Research Group
Research Assistant / Deadline October 17th 2022


 ibecbarcelona.eu
ibecbarcelona.eu