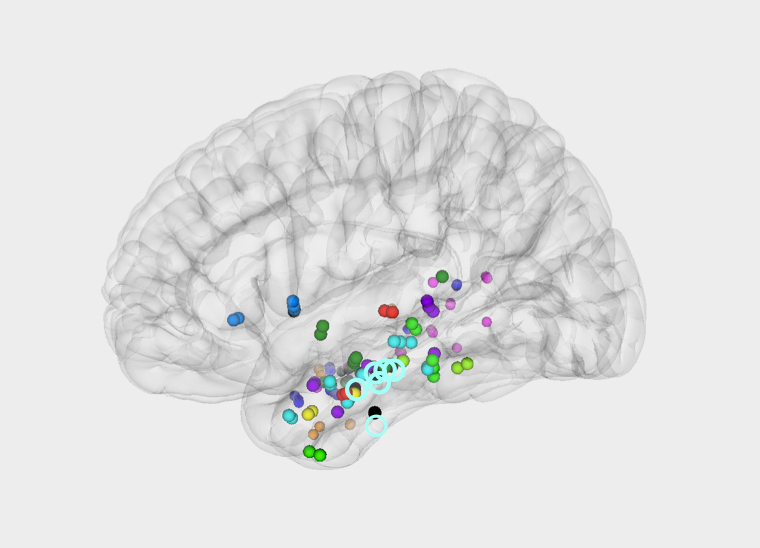
The hippocampus orchestrates the cerebral process that allows us to recall memories
For the first time in humans, researchers from IBEC have simultaneously recorded the brain activity of the two key areas linked to memory: the hippocampus and the neocortex.
This study was made possible thanks to the voluntary participation of epilepsy patients who, due to their disease, have intracranial electrode implants. Making use of virtual reality, the participants performed spatial memory tasks. The taste of your favourite snack after school, your first kiss, that time you partied until dawn… Memories are a way of travelling into the past. Despite how easy it can be to remember a situation, the cerebral process taking place is complex and continues to be, for the most part, a mystery.


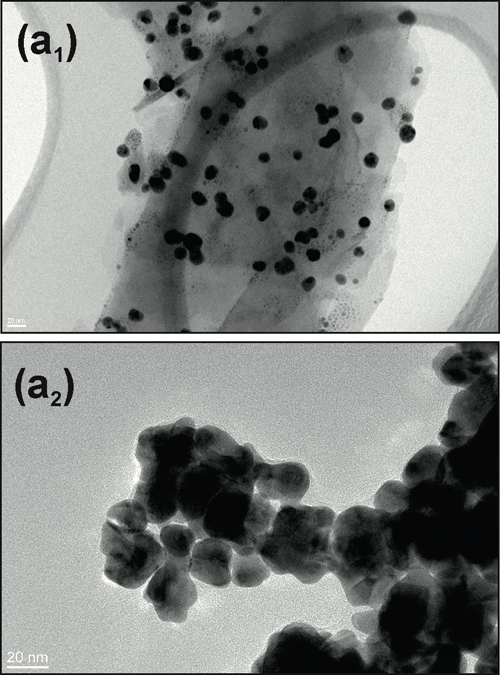
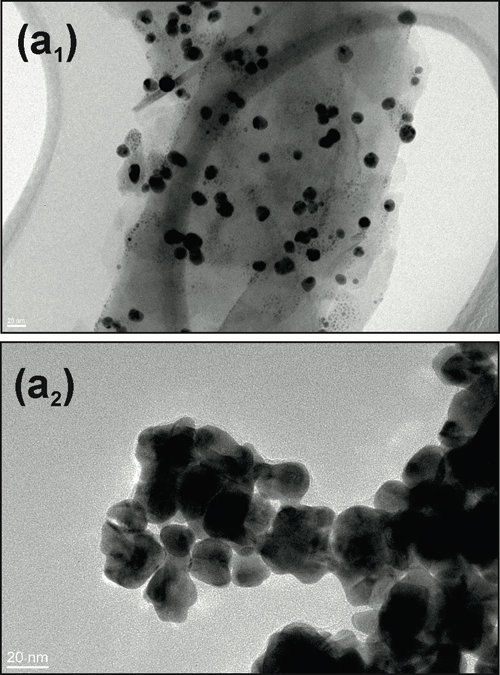 The Bacterial Infections: Antimicrobial Therapies group from IBEC, led by Eduard Torrents, has designed a new method that, for the first time, makes it possible to check antimicrobial treatment efficacy in the presence of nanoparticles.This new technique has recently been published in the Journal of Nanobiotechnology..
The Bacterial Infections: Antimicrobial Therapies group from IBEC, led by Eduard Torrents, has designed a new method that, for the first time, makes it possible to check antimicrobial treatment efficacy in the presence of nanoparticles.This new technique has recently been published in the Journal of Nanobiotechnology..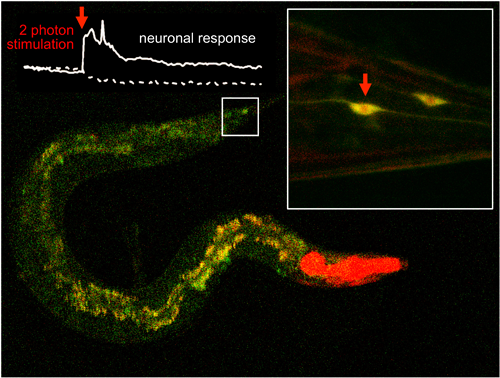
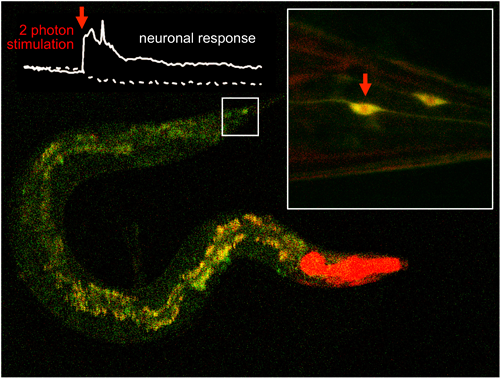 A scientific team led by IBEC and UAB manages to efficiently activate molecules located inside cell tissues using two-photon excitation of with infrared light lasers. The results of the study has been published in Nature Communications.
A scientific team led by IBEC and UAB manages to efficiently activate molecules located inside cell tissues using two-photon excitation of with infrared light lasers. The results of the study has been published in Nature Communications.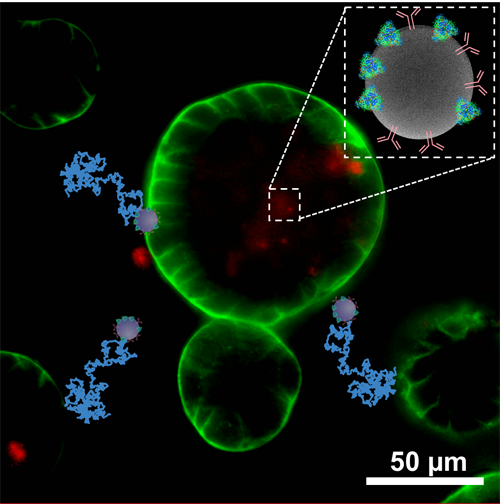
 IBEC’s
IBEC’s 
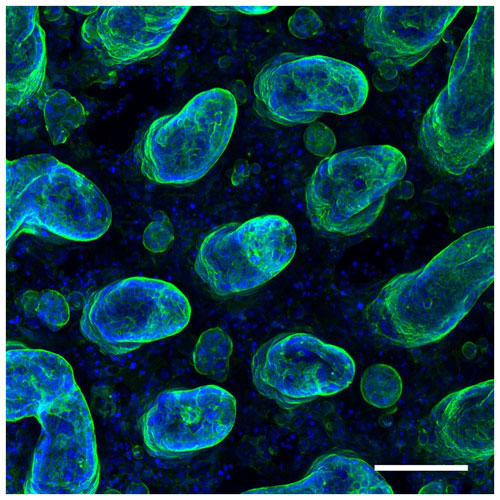
 Researchers from the IBEC have created, for the first time, 3D organoid cultures from pluripotent stem cells, which resemble human embryonic kidney tissue during the second trimester of pregnancy.
Researchers from the IBEC have created, for the first time, 3D organoid cultures from pluripotent stem cells, which resemble human embryonic kidney tissue during the second trimester of pregnancy. 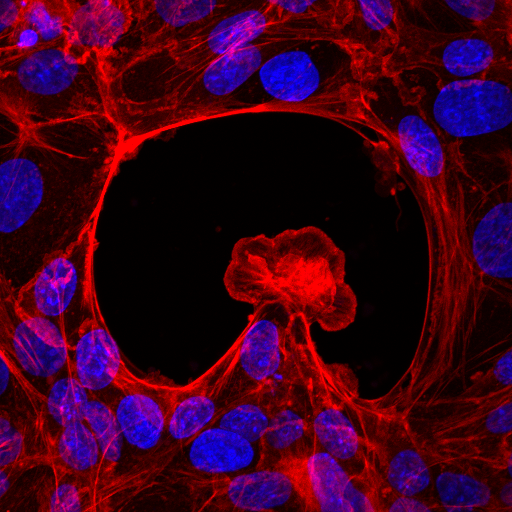
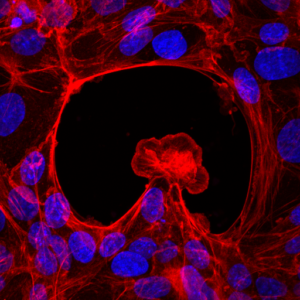
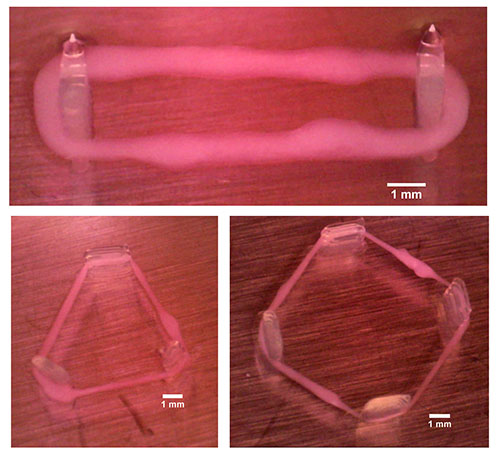
 IBEC’s Smart Nano-Bio-Devices group – the institute’s experts in micro- and nanorobots – have used 3D bioprinting to produce ‘biorobots’ made of biological elements such as muscle tissue.
IBEC’s Smart Nano-Bio-Devices group – the institute’s experts in micro- and nanorobots – have used 3D bioprinting to produce ‘biorobots’ made of biological elements such as muscle tissue. 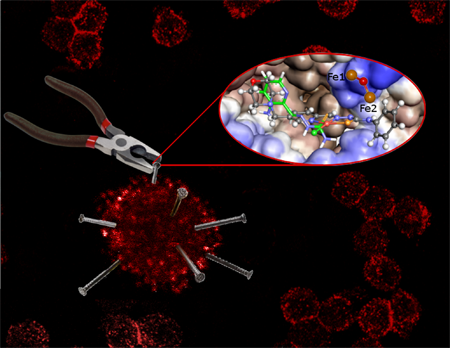
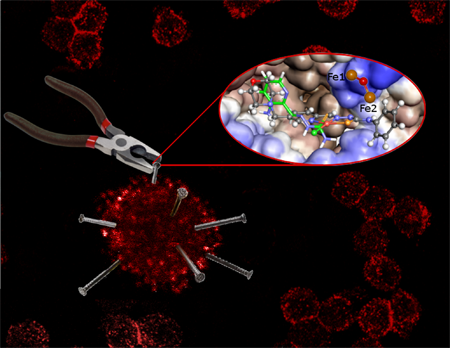 IBEC’s Bacterial infections: antimicrobial therapies group have published two papers offering new hope in the urgent search for antimicrobials.
IBEC’s Bacterial infections: antimicrobial therapies group have published two papers offering new hope in the urgent search for antimicrobials.